Interoperability of RTN1A in dendrite dynamics and immune functions in human Langerhans cells
- PMID: 36223176
- PMCID: PMC9555864
- DOI: 10.7554/eLife.80578
Interoperability of RTN1A in dendrite dynamics and immune functions in human Langerhans cells
Abstract
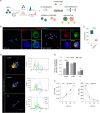
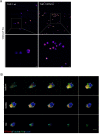
Skin is an active immune organ where professional antigen-presenting cells such as epidermal Langerhans cells (LCs) link innate and adaptive immune responses. While Reticulon 1A (RTN1A) was recently identified in LCs and dendritic cells in cutaneous and lymphoid tissues of humans and mice, its function is still unclear. Here, we studied the involvement of this protein in cytoskeletal remodeling and immune responses toward pathogens by stimulation of Toll-like receptors (TLRs) in resident LCs (rLCs) and emigrated LCs (eLCs) in human epidermis ex vivo and in a transgenic THP-1 RTN1A+ cell line. Hampering RTN1A functionality through an inhibitory antibody induced significant dendrite retraction of rLCs and inhibited their emigration. Similarly, expression of RTN1A in THP-1 cells significantly altered their morphology, enhanced aggregation potential, and inhibited the Ca2+ flux. Differentiated THP-1 RTN1A+ macrophages exhibited long cell protrusions and a larger cell body size in comparison to wild-type cells. Further, stimulation of epidermal sheets with bacterial lipoproteins (TLR1/2 and TLR2 agonists) and single-stranded RNA (TLR7 agonist) resulted in the formation of substantial clusters of rLCs and a significant decrease of RTN1A expression in eLCs. Together, our data indicate involvement of RTN1A in dendrite dynamics and structural plasticity of primary LCs. Moreover, we discovered a relation between activation of TLRs, clustering of LCs, and downregulation of RTN1A within the epidermis, thus indicating an important role of RTN1A in LC residency and maintaining tissue homeostasis.
Keywords: Langerhans cells; RTN1A; Toll-like receptors; cytoskeleton; dendrites; epidermis; human; immunology; inflammation.
© 2022, Cichoń, Cichoń et al.
Conflict of interest statement
MC, KP, JL, LW, CS, PS, AE No competing interests declared
Figures












References
-
- Bernimoulin MP, Zeng XL, Abbal C, Giraud S, Martinez M, Michielin O, Schapira M, Spertini O. Molecular basis of leukocyte rolling on PSGL-1. predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. The Journal of Biological Chemistry. 2003;278:37–47. doi: 10.1074/jbc.M204360200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

