Neuromodulation and the toolkit for behavioural evolution: can ecdysis shed light on an old problem?
- PMID: 36223183
- PMCID: PMC10166064
- DOI: 10.1111/febs.16650
Neuromodulation and the toolkit for behavioural evolution: can ecdysis shed light on an old problem?
Abstract
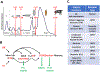
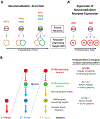
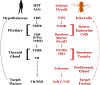
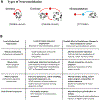
The geneticist Thomas Dobzhansky famously declared: 'Nothing in biology makes sense except in the light of evolution'. A key evolutionary adaptation of Metazoa is directed movement, which has been elaborated into a spectacularly varied number of behaviours in animal clades. The mechanisms by which animal behaviours have evolved, however, remain unresolved. This is due, in part, to the indirect control of behaviour by the genome, which provides the components for both building and operating the brain circuits that generate behaviour. These brain circuits are adapted to respond flexibly to environmental contingencies and physiological needs and can change as a function of experience. The resulting plasticity of behavioural expression makes it difficult to characterize homologous elements of behaviour and to track their evolution. Here, we evaluate progress in identifying the genetic substrates of behavioural evolution and suggest that examining adaptive changes in neuromodulatory signalling may be a particularly productive focus for future studies. We propose that the behavioural sequences used by ecdysozoans to moult are an attractive model for studying the role of neuromodulation in behavioural evolution.
Keywords: Ecdysozoa; Evo-Devo; GPCR; motor programme; neuropeptide.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
Figures





References
-
- Maynard Smith J (1982) Evolution and the theory of games, Cambridge University Press, Cambridge ; New York.
-
- Tinbergen N (1951) The Study of Instinct, Oxford University Press, London.
-
- Shubin N, Tabin C & Carroll S (2009) Deep homology and the origins of evolutionary novelty, Nature. 457, 818–823. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

