Myoglobin regulates fatty acid trafficking and lipid metabolism in mammary epithelial cells
- PMID: 36223378
- PMCID: PMC9555620
- DOI: 10.1371/journal.pone.0275725
Myoglobin regulates fatty acid trafficking and lipid metabolism in mammary epithelial cells
Erratum in
-
Correction: Myoglobin regulates fatty acid trafficking and lipid metabolism in mammary epithelial cells.PLoS One. 2024 Dec 27;19(12):e0316741. doi: 10.1371/journal.pone.0316741. eCollection 2024. PLoS One. 2024. PMID: 39729522 Free PMC article.
Abstract
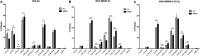
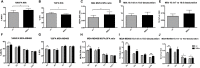
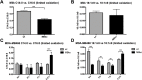
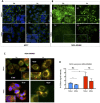
Myoglobin (MB) is known to bind and deliver oxygen in striated muscles at high expression levels. MB is also expressed at much reduced levels in mammary epithelial cells, where the protein´s function is unclear. In this study, we aim to determine whether MB impacts fatty acid trafficking and facilitates aerobic fatty acid ß-oxidation in mammary epithelial cells. We utilized MB-wildtype versus MB-knockout mice and human breast cancer cells to examine the impact of MB and its oxygenation status on fatty acid metabolism in mouse milk and mammary epithelia. MB deficient cells were generated through CRISPR/Cas9 and TALEN approaches and exposed to various oxygen tensions. Fatty acid profiling of milk and cell extracts were performed along with cell labelling and immunocytochemistry. Our findings show that MB expression in mammary epithelial cells promoted fatty acid oxidation while reducing stearyl-CoA desaturase activity for lipogenesis. In cells and milk product, presence of oxygenated MB significantly elevated indices of limited fatty acid ß-oxidation, i.e., the organelle-bound removal of a C2 moiety from long-chain saturated or monounsaturated fatty acids, thus shifting the composition toward more saturated and shorter fatty acid species. Presence of the globin also increased cytoplasmic fatty acid solubility under normoxia and fatty acid deposition to lipid droplets under severe hypoxia. We conclude that MB can function in mammary epithelia as intracellular O2-dependent shuttle of oxidizable fatty acid substrates. MB's impact on limited oxidation of fatty acids could generate inflammatory mediator lipokines, such as 7-hexadecenoate. Thus, the novel functions of MB in breast epithelia described herein range from controlling fatty acid turnover and homeostasis to influencing inflammatory signalling cascade. Future work is needed to analyse to what extent these novel roles of MB also apply to myocytic cell physiology and malignant cell behaviour, respectively.
Conflict of interest statement
NO authors have competing interests.
Figures

References
-
- Tejero J, Kapralov AA, Baumgartner MP, Sparacino-Watkins CE, Anthonymutu TS, Vlasova II, et al.. Peroxidase activation of cytoglobin by anionic phospholipids: Mechanisms and consequences. Biochim Biophys Acta. 2016;1861(5):391–401. Epub 2016/03/02. doi: 10.1016/j.bbalip.2016.02.022 ; PubMed Central PMCID: PMC4821708. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

