1000 spider silkomes: Linking sequences to silk physical properties
- PMID: 36223455
- PMCID: PMC9555773
- DOI: 10.1126/sciadv.abo6043
1000 spider silkomes: Linking sequences to silk physical properties
Abstract
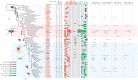
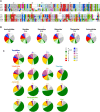
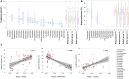
Spider silks are among the toughest known materials and thus provide models for renewable, biodegradable, and sustainable biopolymers. However, the entirety of their diversity still remains elusive, and silks that exceed the performance limits of industrial fibers are constantly being found. We obtained transcriptome assemblies from 1098 species of spiders to comprehensively catalog silk gene sequences and measured the mechanical, thermal, structural, and hydration properties of the dragline silks of 446 species. The combination of these silk protein genotype-phenotype data revealed essential contributions of multicomponent structures with major ampullate spidroin 1 to 3 paralogs in high-performance dragline silks and numerous amino acid motifs contributing to each of the measured properties. We hope that our global sampling, comprehensive testing, integrated analysis, and open data will provide a solid starting point for future biomaterial designs.
Figures

References
-
- Vollrath F., Selden P., The role of behavior in the evolution of spiders, silks, and webs. Annu. Rev. Ecol. Evol. Syst. 38, 819–846 (2007).
-
- Vollrath F., Porter D., Spider silk as archetypal protein elastomer. Soft Matter 2, 377–385 (2006). - PubMed
-
- Fernandez R., Kallal R. J., Dimitrov D., Ballesteros J. A., Arnedo M. A., Giribet G., Hormiga G., Phylogenomics, diversification dynamics, and comparative transcriptomics across the spider tree of life. Curr. Biol. 28, 2190–2193 (2018). - PubMed
-
- Kluge J. A., Rabotyagova O., Leisk G. G., Kaplan D. L., Spider silks and their applications. Trends Biotechnol. 26, 244–251 (2008). - PubMed

