Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227
- PMID: 36223558
- PMCID: PMC9937094
- DOI: 10.1200/JCO.22.01503
Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227
Abstract
Purpose: We present 5-year results from CheckMate 227 Part 1, in which nivolumab plus ipilimumab improved overall survival (OS) versus chemotherapy in patients with metastatic non-small-cell lung cancer, regardless of tumor programmed death ligand 1 (PD-L1) status.
Methods: Adults with stage IV/recurrent non-small-cell lung cancer without EGFR mutations or ALK alterations and with tumor PD-L1 ≥ 1% or < 1% (n = 1739) were randomly assigned. Patients with tumor PD-L1 ≥ 1% were randomly assigned to first-line nivolumab plus ipilimumab, nivolumab alone, or chemotherapy. Patients with tumor PD-L1 < 1% were randomly assigned to nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy. End points included exploratory 5-year results for efficacy, safety, and quality of life.
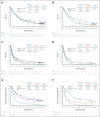
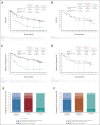
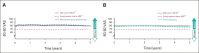
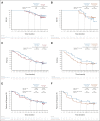
Results: At a minimum follow-up of 61.3 months, 5-year OS rates were 24% versus 14% for nivolumab plus ipilimumab versus chemotherapy (PD-L1 ≥ 1%) and 19% versus 7% (PD-L1 < 1%). The median duration of response was 24.5 versus 6.7 months (PD-L1 ≥ 1%) and 19.4 versus 4.8 months (PD-L1 < 1%). Among patients surviving 5 years, 66% (PD-L1 ≥ 1%) and 64% (PD-L1 < 1%) were off nivolumab plus ipilimumab without initiating subsequent systemic anticancer treatment by the 5-year time point. Survival benefit continued after nivolumab plus ipilimumab discontinuation because of treatment-related adverse events, with a 5-year OS rate of 39% (combined PD-L1 ≥ 1% and < 1% populations). Quality of life in 5-year survivors treated with nivolumab plus ipilimumab was similar to that in the general US population through the 5-year follow-up. No new safety signals were observed.
Conclusion: With all patients off immunotherapy treatment for ≥ 3 years, nivolumab plus ipilimumab increased 5-year survivorship versus chemotherapy, including long-term, durable clinical benefit regardless of tumor PD-L1 expression. These data support nivolumab plus ipilimumab as an effective first-line treatment for patients with metastatic non-small-cell lung cancer.
Trial registration: ClinicalTrials.gov NCT02477826.
Figures
Comment in
-
Who Should Receive the Chemotherapy-Free Combination of Nivolumab Plus Ipilimumab as the First-Line Treatment of Advanced Non-Small-Cell Lung Cancer?J Clin Oncol. 2023 Feb 20;41(6):1172-1175. doi: 10.1200/JCO.22.02278. Epub 2023 Jan 9. J Clin Oncol. 2023. PMID: 36623229 No abstract available.
References
-
- Surveillance, Epidemiology, and End Results Program (SEER) : Cancer Stat Facts: Lung and Bronchus Cancer. http://seer.cancer.gov/statfacts/html/lungb.html
-
- Hanna NH, Temin S, Masters G: Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update summary. JCO Oncol Pract 16:e844-e848, 2020 - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guidelines: Non-Small Cell Lung Cancer V.3.2022. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/...
-
- Planchard D, Popat S, Kerr K, et al. : Metastatic non-small cell lung cancer: ESMO clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-iv237, 2020. (suppl 4) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

