Pim-1 kinase is a positive feedback regulator of the senescent lung fibroblast inflammatory secretome
- PMID: 36223640
- PMCID: PMC9744654
- DOI: 10.1152/ajplung.00023.2022
Pim-1 kinase is a positive feedback regulator of the senescent lung fibroblast inflammatory secretome
Abstract
Cellular senescence is emerging as a driver of idiopathic pulmonary fibrosis (IPF), a progressive and fatal disease with limited effective therapies. The senescence-associated secretory phenotype (SASP), involving the release of inflammatory cytokines and profibrotic growth factors by senescent cells, is thought to be a product of multiple cell types in IPF, including lung fibroblasts. NF-κB is a master regulator of the SASP, and its activity depends on the phosphorylation of p65/RelA. The purpose of this study was to assess the role of Pim-1 kinase as a driver of NF-κB-induced production of inflammatory cytokines from low-passage IPF fibroblast cultures displaying markers of senescence. Our results demonstrate that Pim-1 kinase phosphorylates p65/RelA, activating NF-κB activity and enhancing IL-6 production, which in turn amplifies the expression of PIM1, generating a positive feedback loop. In addition, targeting Pim-1 kinase with a small molecule inhibitor dramatically inhibited the expression of a broad array of cytokines and chemokines in IPF-derived fibroblasts. Furthermore, we provide evidence that Pim-1 overexpression in low-passage human lung fibroblasts is sufficient to drive premature senescence, in vitro. These findings highlight the therapeutic potential of targeting Pim-1 kinase to reprogram the secretome of senescent fibroblasts and halt IPF progression.
Keywords: Pim kinase; idiopathic pulmonary fibrosis; lung fibrosis; secretome; senescence.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


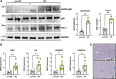
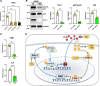


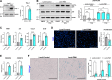
Similar articles
-
Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation.Aging Cell. 2019 Feb;18(1):e12858. doi: 10.1111/acel.12858. Epub 2018 Dec 12. Aging Cell. 2019. PMID: 30548445 Free PMC article.
-
CD148 Deficiency in Fibroblasts Promotes the Development of Pulmonary Fibrosis.Am J Respir Crit Care Med. 2021 Aug 1;204(3):312-325. doi: 10.1164/rccm.202008-3100OC. Am J Respir Crit Care Med. 2021. PMID: 33784491 Free PMC article.
-
Senescent lung fibroblasts in idiopathic pulmonary fibrosis facilitate non-small cell lung cancer progression by secreting exosomal MMP1.Oncogene. 2025 Mar;44(11):769-781. doi: 10.1038/s41388-024-03236-5. Epub 2024 Dec 11. Oncogene. 2025. PMID: 39663393 Free PMC article.
-
Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2018 Aug 1;315(2):L162-L172. doi: 10.1152/ajplung.00037.2018. Epub 2018 Apr 26. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29696986 Free PMC article. Review.
-
PI3K/Akt in IPF: untangling fibrosis and charting therapies.Front Immunol. 2025 Mar 31;16:1549277. doi: 10.3389/fimmu.2025.1549277. eCollection 2025. Front Immunol. 2025. PMID: 40248697 Free PMC article. Review.
Cited by
-
PIM1 signaling in immunoinflammatory diseases: an emerging therapeutic target.Front Immunol. 2024 Sep 20;15:1443784. doi: 10.3389/fimmu.2024.1443784. eCollection 2024. Front Immunol. 2024. PMID: 39372407 Free PMC article. Review.
-
Differential susceptibility and role for senescence in CART cells based on costimulatory domains.Mol Cancer. 2025 Jun 10;24(1):172. doi: 10.1186/s12943-025-02371-1. Mol Cancer. 2025. PMID: 40495168 Free PMC article.
-
Cellular Senescence: A Troy Horse in Pulmonary Fibrosis.Int J Mol Sci. 2023 Nov 16;24(22):16410. doi: 10.3390/ijms242216410. Int J Mol Sci. 2023. PMID: 38003600 Free PMC article. Review.
-
NR2F2 alleviates pulmonary fibrosis by inhibition of epithelial cell senescence.Respir Res. 2024 Apr 2;25(1):154. doi: 10.1186/s12931-024-02777-3. Respir Res. 2024. PMID: 38566093 Free PMC article.
-
Development and Application of a Senolytic Predictor for Discovery of Novel Senolytic Compounds and Herbs.Molecules. 2025 Jun 19;30(12):2653. doi: 10.3390/molecules30122653. Molecules. 2025. PMID: 40572616 Free PMC article.
References
-
- Glassberg MK. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am J Manag Care 25: S195–S203, 2019. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

