Clinical relevance of single-subject brain metabolism patterns in amyotrophic lateral sclerosis mutation carriers
- PMID: 36223668
- PMCID: PMC9668615
- DOI: 10.1016/j.nicl.2022.103222
Clinical relevance of single-subject brain metabolism patterns in amyotrophic lateral sclerosis mutation carriers
Abstract
Background and objectives: The ALS diagnosis requires an integrative approach, combining the clinical examination and supporting tests. Nevertheless, in several cases, the diagnosis proves to be suboptimal, and for this reason, new diagnostic methods and novel biomarkers are catching on. The 18F-fluorodeoxyglucose (18F-FDG)-PET could be a helpful method, but it still requires additional research for sensitivity and specificity. We performed an 18F-FDG-PET single-subject analysis in a sample of familial ALS patients carrying different gene mutations, investigating the genotype-phenotype correlations and exploring metabolism correlations with clinical and neuropsychological data.
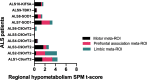
Methods: We included ten ALS patients with pathogenic gene mutation who underwent a complete clinical and neuropsychological evaluation and an 18F-FDG-PET scan at baseline. Patients were recruited between 2018 and 2022 at the ALS Tertiary Centre in Novara, Italy. Patients were selected based on the presence of ALS gene mutation (C9orf72, SOD1, TBK1, and KIF5A). Following a validated voxel-based Statistical Parametric Mapping (SPM) procedure, we obtained hypometabolism maps at single-subject level. We extracted regional hypometabolism from the SPM maps, grouping significant hypometabolism regions into three meta-ROIs (motor, prefrontal association and limbic). Then, the corresponding 18F-FDG-PET regional hypometabolism was correlated with clinical and neuropsychological features.
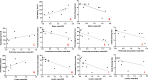
Results: Classifying the patients with C9orf72-ALS based on the rate of disease progression from symptoms onset to the time of scan, we observed two different patterns of brain hypometabolism: an extensive motor and prefrontal hypometabolism in patients classified as fast progressors, and a more limited brain hypometabolism in patients grouped as slow progressors. Patients with SOD1-ALS showed a hypometabolic pattern involving the motor cortex and prefrontal association regions, with a minor involvement of the limbic regions. The patient with TBK1-ALS showed an extended hypometabolism, in limbic systems, along with typical motor involvement, while the hypometabolism in the patient with KIF5A-ALS involved almost exclusively the motor regions, supporting the predominantly motor impairment linked to this gene mutation. Additionally, we observed strong correlations between the hypometabolism in the motor, prefrontal association and limbic meta-ROI and the specific neuropsychological performances.
Conclusions: To our knowledge, this is the first study investigating brain hypometabolism at the single-subject level in genetic ALS patients carrying different mutations. Our results show high heterogeneity in the hypometabolism maps and some commonalities in groups sharing the same mutation. Specifically, in patients with C9orf72-ALS the brain hypometabolism was larger in patients classified as fast progressors than slow progressors. In addition, in the whole group, the brain metabolism showed specific correlations with clinical and neuropsychological impairment, confirming the ability of 18F-FDG-PET in revealing pattern of neuronal dysfunction, aiding the diagnostic workup in genetic ALS patients.
Keywords: (18)F-FDG-PET; Amyotrophic lateral sclerosis; Brain metabolism; Genetic; Neurodegenerative diseases; Positron emission tomography.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Heterogeneous brain FDG-PET metabolic patterns in patients with C9orf72 mutation.Neurol Sci. 2019 Mar;40(3):515-521. doi: 10.1007/s10072-018-3685-7. Epub 2018 Dec 15. Neurol Sci. 2019. PMID: 30554355
-
Coupling motor evoked potentials and brain [18F]FDG-PET in Amyotrophic Lateral Sclerosis: preliminary findings on disease severity.Neurobiol Dis. 2024 Sep;199:106579. doi: 10.1016/j.nbd.2024.106579. Epub 2024 Jun 25. Neurobiol Dis. 2024. PMID: 38936435
-
Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study.JAMA Neurol. 2014 May;71(5):553-61. doi: 10.1001/jamaneurol.2014.62. JAMA Neurol. 2014. PMID: 24615479
-
Clinical utility of FDG-PET in amyotrophic lateral sclerosis and Huntington's disease.Eur J Nucl Med Mol Imaging. 2018 Jul;45(9):1546-1556. doi: 10.1007/s00259-018-4033-0. Epub 2018 May 1. Eur J Nucl Med Mol Imaging. 2018. PMID: 29717332 Review.
-
Positron emission tomography neuroimaging in amyotrophic lateral sclerosis: what is new?Q J Nucl Med Mol Imaging. 2014 Dec;58(4):344-54. Epub 2014 Nov 6. Q J Nucl Med Mol Imaging. 2014. PMID: 25375229 Review.
Cited by
-
Potential of neuroimaging as a biomarker in amyotrophic lateral sclerosis: from structure to metabolism.J Neurol. 2024 May;271(5):2238-2257. doi: 10.1007/s00415-024-12201-x. Epub 2024 Feb 17. J Neurol. 2024. PMID: 38367047 Review.
-
The Need for Biomarkers in the ALS-FTD Spectrum: A Clinical Point of View on the Role of Proteomics.Proteomes. 2023 Jan 9;11(1):1. doi: 10.3390/proteomes11010001. Proteomes. 2023. PMID: 36648959 Free PMC article. Review.
-
Association of Reduced Brain Metabolism With Motor Function and Survival in Amyotrophic Lateral Sclerosis Patients With Neurofilament Heavy (NEFH) Gene Mutation.Eur J Neurol. 2025 Jul;32(7):e70261. doi: 10.1111/ene.70261. Eur J Neurol. 2025. PMID: 40607881 Free PMC article.
-
Neuroinflammatory Pathways in the ALS-FTD Continuum: A Focus on Genetic Variants.Genes (Basel). 2023 Aug 21;14(8):1658. doi: 10.3390/genes14081658. Genes (Basel). 2023. PMID: 37628709 Free PMC article. Review.
-
The Differential Effects of Genetic Mutations in ALS and FTD Genes on Behavioural and Cognitive Changes: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2025 Jun 27;26(13):6199. doi: 10.3390/ijms26136199. Int J Mol Sci. 2025. PMID: 40649976 Free PMC article. Review.
References
-
- Agosta F., Al-Chalabi A., Filippi M., Hardiman O., Kaji R., Meininger V., Nakano I., Shaw P., Shefner J., Van Den Berg L.H. The El Escorial criteria: strengths and weaknesses. Amyotroph. Lateral Scler. Front. Degener. 2015;16:1–7. - PubMed
-
- Agosta F., Altomare D., Festari C., Orini S., Gandolfo F., Boccardi M., Arbizu J., Bouwman F., Drzezga A., Nestor P. Clinical utility of FDG-PET in amyotrophic lateral sclerosis and Huntington’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1546–1556. - PubMed
-
- Boeve B.F., Boylan K.B., Graff-Radford N.R., DeJesus-Hernandez M., Knopman D.S., Pedraza O., Vemuri P., Jones D., Lowe V., Murray M.E., Dickson D.W., Josephs K.A., Rush B.K., Machulda M.M., Fields J.A., Ferman T.J., Baker M., Rutherford N.J., Adamson J., Wszolek Z.K., Adeli A., Savica R., Boot B., Kuntz K.M., Gavrilova R., Reeves A., Whitwell J., Kantarci K., Jack C.R.J., Parisi J.E., Lucas J.A., Petersen R.C., Rademakers R. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

