Subcutaneous injection of trastuzumab into the thigh versus abdominal wall in patients with HER2-positive early breast cancer: Pharmacokinetic, safety and patients' preference - Substudy of the randomised phase III GAIN-2 study
- PMID: 36223695
- PMCID: PMC9563210
- DOI: 10.1016/j.breast.2022.10.002
Subcutaneous injection of trastuzumab into the thigh versus abdominal wall in patients with HER2-positive early breast cancer: Pharmacokinetic, safety and patients' preference - Substudy of the randomised phase III GAIN-2 study
Abstract
Background: Trastuzumab given intravenously in combination with chemotherapy is standard of care for patients with early HER2-positive breast cancer (BC). Different randomised studies have shown equivalent efficacy of a subcutaneous injection into the thigh compared to the intravenous formulation. Other body regions for injection have not been investigated but might be more convenient for patients.
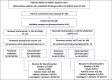
Methods: After surgery, patients were randomised to receive either subcutaneous trastuzumab into the thigh or into the abdominal wall (AW). Patient preferences were evaluated using validated questionnaires (PINT). Primary objectives of this multicentre, non-blinded, randomised substudy of the GAIN-2 study were to investigate pharmacokinetics of the injection into the thigh versus AW and to determine patients' preferences of either administration site versus the previously received intravenous application.
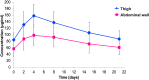
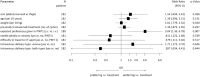
Results: 226 patients were randomised and 219 patients (thigh: N = 110; AW: N = 109) formed the modified intent-to-treat (mITT). Overall, 83.5% (out of N = 182 with information about patients' preference) preferred subcutaneous over previous intravenous application or had no preference. Preference was similar between both administration sites (thigh: 80.6%; AW: 86.5; p = 0.322). Pharmacokinetic analysis included 30 patients. Geometric means of Cmax and AUC0-21d were higher in thigh than in AW group (geometric mean ratio with body weight adjustment: Cmax: 1.291, 90%-CI 1.052-1.584; AUC0-21d: 1.291, 90%-CI 1.026-1.626). Safety profile was in line with previous reports of subcutaneous trastuzumab.
Conclusion: Subcutaneous trastuzumab into the thigh showed an approximately 30% higher bioavailability. Injections were well tolerated and preferred over intravenous administration. The subcutaneous injection into the thigh should remain the standard of care.
Keywords: Breast cancer; Pharmacokinetic; Safety; Thigh or abdominal wall; Trastuzumab subcutaneous; patients' preference.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest MR reports travel support from AstraZeneca, Celgene, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Seagen, Somatex and Roche. MU reports personal fees for lectures and/or consultancy from Abbvie, Amgen, Astra Zeneca, BMS, Celgene, Daiji Sankyo, Gilead, GSK, Lilly, MSD Merck, Mundipharma, Myriad Genetics, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi Aventis, Saegen. MS reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche, and SeaGen outside the submitted work. Institutional research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen; has a patent for EP2390370 B1 issued and a patent for EP2951317 B1issued. SL reports other from Amgen, other from BMS, grants and other from Celgene, grants, non-financial support and other from Roche, during the conduct of the study; grants and other from Abbvie, grants and other from AstraZeneca, other from Eirgenix, other from GSK, grants, non-financial support and other from Gilead, other from Lilly, other from Merck, grants, non-financial support and other from Novartis, grants, non-financial support and other from Pfizer, other from Pierre Fabre, non-financial support and other from Seagen, grants, non-financial support and other from Daiichi-Sankyo, other from Sanofi, outside the submitted work; In addition; has a patent EP14153692.0 pending, a patent EP21152186.9 pending, a patent EP15702464.7 issued, a patent EP19808852.8 pending, and a patent Digital Ki67 Evaluator with royalties paid. ES reports honoraria from Pfizer, Roche, AstraZeneca, MSD, Gilead, Daiichi-Sankyo, Novartis, Seagen, Lilly, Pierre Fabre. SaS reports grants and non-financial support from F. Hoffman-La Roche, grants from BMS (Celgene), Amgen, during the conduct of the study; personal fees from Abbvie, outside the submitted work. CJ reports Honoraria from Amgen, AstraZeneca, Roche, Lilly, Novartis, Pfizer, Exact Sciences, Pierre-Fabré, Molecular Health. FM reports personal fees from Roche, AstraZeneca, Pfizer, Tesaro, Novartis, Amgen, PharmaMar, GenomicHealth, CureVac, EISAI outside the submitted work. CD reports stock and other ownership interest from Sividon Diagnostics (until 2016); consulting or advisor role from MSD Oncology, Daiichi Sankyo, Molecular Health, AstraZeneca, Merck, Roche, Lilly; research funding from Myriad Genetics, Roche; patents, royalties or other intellectual property from VMScope digital pathology software, patent applications WO2015114146A1 and WO2010076322A1-therapy response, patent application WO2020109570A1-immunotherapy. No other potential conflict of interest relevant to this article was reported.
Figures
References
-
- Lurie R.H., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. 2020. NCCN guidelines version 3.2020 breast cancer. - DOI
-
- von Minckwitz G., Untch M., Blohmer J.-U., Costa S.D., Eidtmann H., Fasching P.A., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

