Longitudinal COVID-19 immune trajectories in patients with neurological autoimmunity on anti-CD20 therapy
- PMID: 36223705
- PMCID: PMC9511881
- DOI: 10.1016/j.msard.2022.104195
Longitudinal COVID-19 immune trajectories in patients with neurological autoimmunity on anti-CD20 therapy
Abstract
Background and objectives: During the COVID-19 pandemic, B cell depleting therapies pose a clinical concern for patients with neuroimmune conditions, as patients may not mount a sufficient immune response to SARS-CoV-2 infection and vaccinations. Studies to-date have reported conflicting results on the degree of antibody production post-SARS-CoV-2 infection and vaccinations in B cell depleted patients, focusing primarily on short-term immune profiling. Our objective was to follow longitudinal immune responses in COVID-19 B cell depleted patients with neuroimmune disorders post-COVID-19 and SARS-CoV-2-vaccination.
Methods: CD20 B cell depleted autoimmune patients and age/sex-matched controls positive for SARS-CoV-2 were recruited at Dell Medical School, UT Austin between 2020 and 2021, followed prospectively for 12 months and evaluated at multiple time points for spike S1 receptor binding domain (RBD) antibody titers, B and T cell composition, and frequency of T cells specific for SARS-CoV-2 antigens.
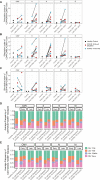
Results: Immune responses post-SARS-CoV-2 infection and vaccination were evaluated in a cohort of COVID-19 B cell depleted neuroimmune patients (n = 5), COVID-19 non-B cell depleted autoimmune patients (n = 15), COVID-19 immunocompetent patients (n = 117), and healthy controls (n = 6) for a total of 259 samples in 137 participants. 4/5 B cell-depleted patients developed detectable anti-spike RBD antibodies, which were boosted by vaccination in 2 patients. While spike RBD antibodies were associated with presence of CD20+ B cells, very few B cells were required. In contrast, patients whose B cell compartment primarily consisted of CD19+CD20- Bcells during acute COVID-19 disease or vaccination did not seroconvert. Interestingly, circulating Bcells in B cell depleted patients were significantly CD38high with co-expression of CD24 and CD27, indicating that B cell depletion may impact B cell activation patterns. Additionally, all B cell depleted patients mounted a sustained T cell response to SARS-CoV-2 antigens, regardless of seroconversion. Specifically, all patients developed naïve, central memory, effector memory, and effector memory RA+ T cells, suggesting intact T cell memory conversion in B cell depleted patients compared to controls.
Discussion: We present the longest COVID-19 immune profiling analysis to date in B cell depleted patients, demonstrating that both humoral and cellular immune responses can be generated and sustained up to 12 months post SARS-CoV-2 infection and vaccination. Notably, failure to establish humoral immunity did not result in severe disease. We also highlight specific T and B cell signatures that could be used as clinical biomarkers to advise patients on timing of SARS-CoV-2 vaccinations.
Keywords: COVID-19 CD20 B cell depletion therapy Multiple Sclerosis Autoimmune.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest Sam Bazzi: Nothing to disclose Cole Maguire: Nothing to disclose Kerin Hurley: Nothing to disclose Janelle Geltman: Nothing to disclose Lauren Ehrlich: Nothing to disclose Todd Triplett: Funding from OnKure Esther Melamed: has received research funding from Babson Diagnostics, honorarium from Multiple Sclerosis Association of America and has served on advisory boards of Genentech, Horizon, Teva and Viela Bio.
Figures





References
-
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. - PMC - PubMed
-
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., E Markowitz C., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. - PMC - PubMed
-
- Bautista D., Vásquez C., Ayala-Ramírez P., Téllez-Sosa J., Godoy-Lozano E., Martínez-Barnetche J., Franco M., Angel J. Differential expression of IgM and IgD discriminates two subpopulations of human circulating IgM+IgD+CD27+ B cells that differ phenotypically, functionally, and genetically. Front. Immunol. 2020;11 - PMC - PubMed
-
- Bryl E. B cells as target for immunotherapy in rheumatic diseases–current status. Immunol. Lett. 2021;236:12–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

