Tripartite antigen-agnostic combination immunotherapy cures established poorly immunogenic tumors
- PMID: 36223955
- PMCID: PMC9562723
- DOI: 10.1136/jitc-2022-004781
Tripartite antigen-agnostic combination immunotherapy cures established poorly immunogenic tumors
Abstract
Background: Single-agent immunotherapy has shown remarkable efficacy in selected cancer entities and individual patients. However, most patients fail to respond. This is likely due to diverse immunosuppressive mechanisms acting in a concerted way to suppress the host anti-tumor immune response. Combination immunotherapy approaches that are effective in such poorly immunogenic tumors mostly rely on precise knowledge of antigenic determinants on tumor cells. Creating an antigen-agnostic combination immunotherapy that is effective in poorly immunogenic tumors for which an antigenic determinant is not known is a major challenge.
Methods: We use multiple cell line and poorly immunogenic syngeneic, autochthonous, and autologous mouse models to evaluate the efficacy of a novel combination immunotherapy named tripartite immunotherapy (TRI-IT). To elucidate TRI-ITs mechanism of action we use immune cell depletions and comprehensive tumor and immune infiltrate characterization by flow cytometry, RNA sequencing and diverse functional assays.
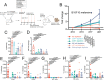
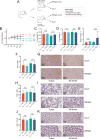
Results: We show that combined adoptive cellular therapy (ACT) with lymphokine-activated killer cells, cytokine-induced killer cells, Vγ9Vδ2-T-cells (γδ-T-cells) and T-cells enriched for tumor recognition (CTLs) display synergistic antitumor effects, which are further enhanced by cotreatment with anti-PD1 antibodies. Most strikingly, the full TRI-IT protocol, a combination of this ACT with anti-PD1 antibodies, local immunotherapy of agonists against toll-like receptor 3, 7 and 9 and pre-ACT lymphodepletion, eradicates and induces durable anti-tumor immunity in a variety of poorly immunogenic syngeneic, autochthonous, as well as autologous humanized patient-derived models. Mechanistically, we show that TRI-IT coactivates adaptive cellular and humoral, as well as innate antitumor immune responses to mediate its antitumor effect without inducing off-target toxicity.
Conclusions: Overall, TRI-IT is a novel, highly effective, antigen-agnostic, non-toxic combination immunotherapy. In this study, comprehensive insights into its preclinical efficacy, even in poorly immunogenic tumors, and mode of action are given, so that translation into clinical trials is the next step.
Keywords: immunotherapy; lung neoplasms; melanoma; sarcoma.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SB has received travel support from BMS and travel support and consulting fees from Takeda. SB received research funding form Takeda, but this funding did not support the research described in this paper. DK is a cofounder of XRAD Therapeutics, which is developing radiosensitizers. DK is the recipient of a Stand Up To Cancer (SU2C) Merck Catalyst Grant studying pembrolizumab and radiation therapy in sarcoma patients. DK has received research funding from XRAD Therapeutics, Eli Lilly & Co., Bristol Myers Squibb, Varian Medical Systems, and Merck, but this funding did not support the research described in this paper. CR received consulting and lecture fees from Abbvie, Astra-Zeneca, Vertex and Merck. CR received research funding from Gilead Pharmaceuticals. CR is a cofounder of CDL Therapeutics.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
