Stretch reflex gain scaling at the shoulder varies with synergistic muscle activity
- PMID: 36224165
- PMCID: PMC9662809
- DOI: 10.1152/jn.00259.2022
Stretch reflex gain scaling at the shoulder varies with synergistic muscle activity
Abstract
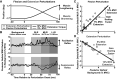
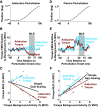
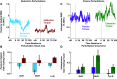
The unique anatomy of the shoulder allows for expansive mobility but also sometimes precarious stability. It has long been suggested that stretch-sensitive reflexes contribute to maintaining joint stability through feedback control, but little is known about how stretch-sensitive reflexes are coordinated between the muscles of the shoulder. The purpose of this study was to investigate the coordination of stretch reflexes in shoulder muscles elicited by rotations of the glenohumeral joint. We hypothesized that stretch reflexes are sensitive to not only a given muscle's background activity but also the aggregate activity of all muscles crossing the shoulder based on the different groupings of muscles required to actuate the shoulder in three rotational degrees of freedom. We examined the relationship between a muscle's background activity and its reflex response in eight shoulder muscles by applying rotational perturbations while participants produced voluntary isometric torques. We found that this relationship, defined as gain scaling, differed at both short and long latencies based on the direction of voluntary torque generated by the participant. Therefore, gain scaling differed based on the aggregate of muscles that were active, not just the background activity in the muscle within which the reflex was measured. Across all muscles, the consideration of torque-dependent gain scaling improved model fits (ΔR2) by 0.17 ± 0.12. Modulation was most evident when volitional torques and perturbation directions were aligned along the same measurement axis, suggesting a functional role in resisting perturbations among synergists while maintaining task performance.NEW & NOTEWORTHY Careful coordination of muscles crossing the shoulder is needed to maintain the delicate balance between the joint's mobility and stability. We provide experimental evidence that stretch reflexes within shoulder muscles are modulated based on the aggregate activity of muscles crossing the joint, not just the activity of the muscle in which the reflex is elicited. Our results reflect coordination through neural coupling that may help maintain shoulder stability during encounters with environmental perturbations.
Keywords: gain scaling; reflex coordination; shoulder; stretch reflex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Translations of the Humeral Head Elicit Reflexes in Rotator Cuff Muscles That Are Larger Than Those in the Primary Shoulder Movers.Front Integr Neurosci. 2022 Feb 2;15:796472. doi: 10.3389/fnint.2021.796472. eCollection 2021. Front Integr Neurosci. 2022. PMID: 35185484 Free PMC article.
-
Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes.Exp Brain Res. 1999 Nov;129(1):93-113. doi: 10.1007/s002210050940. Exp Brain Res. 1999. PMID: 10550507
-
Contributions of altered stretch reflex coordination to arm impairments following stroke.J Neurophysiol. 2010 Dec;104(6):3612-24. doi: 10.1152/jn.00804.2009. Epub 2010 Oct 20. J Neurophysiol. 2010. PMID: 20962072 Free PMC article.
-
Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability.Clin Neurophysiol. 2010 Oct;121(10):1680-9. doi: 10.1016/j.clinph.2010.02.166. Clin Neurophysiol. 2010. PMID: 20434396 Free PMC article. Review.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
Cited by
-
Assessing shoulder muscle stretch reflexes following breast cancer treatment and postmastectomy breast reconstruction.J Neurophysiol. 2023 Apr 1;129(4):914-926. doi: 10.1152/jn.00081.2022. Epub 2023 Mar 22. J Neurophysiol. 2023. PMID: 36947887 Free PMC article.
-
Cancer survivors post-chemotherapy exhibit unimpaired short-latency stretch reflexes in the proximal upper extremity.J Neurophysiol. 2023 Oct 1;130(4):895-909. doi: 10.1152/jn.00299.2022. Epub 2023 Sep 6. J Neurophysiol. 2023. PMID: 37671425 Free PMC article.
-
Feedback parameters for a closed-loop multiple-input multiple-output model of the upper limb.PLoS Comput Biol. 2025 Jun 30;21(6):e1013183. doi: 10.1371/journal.pcbi.1013183. eCollection 2025 Jun. PLoS Comput Biol. 2025. PMID: 40587570 Free PMC article.
-
Assistive Loading Promotes Goal-Directed Tuning of Stretch Reflex Gains.eNeuro. 2023 Feb 27;10(2):ENEURO.0438-22.2023. doi: 10.1523/ENEURO.0438-22.2023. Print 2023 Feb. eNeuro. 2023. PMID: 36781230 Free PMC article.
-
What keeps a shoulder stable - Is there an ideal method for anterior stabilisation?Shoulder Elbow. 2024 Feb;16(1):4-7. doi: 10.1177/17585732231224699. Epub 2024 Jan 9. Shoulder Elbow. 2024. PMID: 38435031 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

