A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer
- PMID: 36224176
- PMCID: PMC9556512
- DOI: 10.1038/s41467-022-33267-z
A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer
Abstract
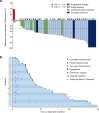
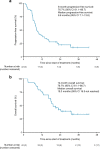
In this multi-center phase II trial, we evaluated the efficacy and safety of a quadruplet regimen (pembrolizumab, trastuzumab, and doublet chemotherapy) as first-line therapy for unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (AGC) (NCT02901301). The primary endpoints were recommended phase 2 dose (RP2D) for phase Ib and objective response rate (ORR) for phase II. The secondary endpoints included progression-free survival (PFS), overall survival (OS), duration of response, time to response and safety. Without dose-limiting or unexpected toxicities, the starting dose in the phase Ib trial was selected as RP2D. In 43 patients, the primary endpoint was achieved: the objective response rate was 76.7% (95% confidence interval [CI]: 61.4-88.2), with complete and partial responses in 14% and 62.8% of patients, respectively. The median progression-free survival, overall survival, and duration of response were 8.6 months, 19.3 months, and 10.8 months, respectively. No patients discontinued pembrolizumab because of immune-related adverse events. Programmed death ligand-1 status was not related to survival. Post hoc analyses of pretreatment tumor specimens via targeted sequencing indicated that ERBB2 amplification, RTK/RAS pathway alterations, and high neoantigen load corrected by HLA-B were positively related to survival. The current quadruplet regimen shows durable efficacy and safety for patients with HER2-positive AGC.
© 2022. The Author(s).
Conflict of interest statement
S.Y.R. received grant/research support from MSD, Celltrion, Boehringer-Ingelheim, Eli Lilly, Taiho, Bristol-Myers Squibb, ASLAN, and Incyte; consultation fees for Daiichi Sankyo, MSD, Eli Lilly, Bristol-Myers Squibb, and Eisail; and served on a speaker’s bureau for Eli Lilly, Bristol-Myers Squibb, and MSD outside the submitted work. H.C.C. received grants/research support from Eli Lilly, Glaxo SmithKline, MSD, Merck-Serono, Bristol-Myers Squibb, Taiho, Amgen, Beigene, Incyte, and Zymework; honoraria from Merck-Serono and Eli Lilly; and consultation fees from Taiho, Celltrion, MSD, Eli Lilly, Bristol-Myers Squibb, Merck-Serono, Gloria, Beigene, Amgen, and Zymework outside the submitted work. All remaining authors declare no competing interests.
Figures



References
-
- Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

