Rapid joule heating improves vitrification based cryopreservation
- PMID: 36224179
- PMCID: PMC9556611
- DOI: 10.1038/s41467-022-33546-9
Rapid joule heating improves vitrification based cryopreservation
Abstract
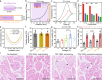
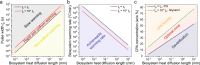
Cryopreservation by vitrification has far-reaching implications. However, rewarming techniques that are rapid and scalable (both in throughput and biosystem size) for low concentrations of cryoprotective agent (CPA) for reduced toxicity are lacking, limiting the potential for translation. Here, we introduce a joule heating-based platform technology, whereby biosystems are rapidly rewarmed by contact with an electrical conductor that is fed a voltage pulse. We demonstrate successful cryopreservation of three model biosystems with thicknesses across three orders of magnitude, including adherent cells (~4 µm), Drosophila melanogaster embryos (~50 µm) and rat kidney slices (~1.2 mm) using low CPA concentrations (2-4 M). Using tunable voltage pulse widths from 10 µs to 100 ms, numerical simulation predicts that warming rates from 5 × 104 to 6 × 108 °C/min can be achieved. Altogether, our results present a general solution to the cryopreservation of a broad spectrum of cellular, organismal and tissue-based biosystems.
© 2022. The Author(s).
Conflict of interest statement
The authors (L.Z., Z.H., Q.S., M.E., and J.B.) have filed a provisional patent application (serial no. 63/358,377) related to this work. The remaining author declares no competing interests.
Figures




Similar articles
-
Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells.Sci Rep. 2020 Aug 12;10(1):13605. doi: 10.1038/s41598-020-70707-6. Sci Rep. 2020. PMID: 32788637 Free PMC article.
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hUCM-MSCs cryopreserved by vitrification.Acta Biomater. 2016 Mar;33:264-74. doi: 10.1016/j.actbio.2016.01.026. Epub 2016 Jan 21. Acta Biomater. 2016. PMID: 26802443 Free PMC article.
-
Optimal vitrification protocol for mouse ovarian tissue cryopreservation: effect of cryoprotective agents and in vitro culture on vitrified-warmed ovarian tissue survival.Hum Reprod. 2014 Apr;29(4):720-30. doi: 10.1093/humrep/det449. Epub 2013 Dec 22. Hum Reprod. 2014. PMID: 24365801
-
Parasite cryopreservation by vitrification.Cryobiology. 2004 Dec;49(3):201-10. doi: 10.1016/j.cryobiol.2004.09.002. Cryobiology. 2004. PMID: 15615606 Review.
Cited by
-
Ice formation and its elimination in cryopreservation of oocytes.Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. Sci Rep. 2024. PMID: 39138273 Free PMC article.
-
Functional Materials and Innovative Strategies for Wearable Thermal Management Applications.Nanomicro Lett. 2023 Jun 29;15(1):160. doi: 10.1007/s40820-023-01126-1. Nanomicro Lett. 2023. PMID: 37386321 Free PMC article. Review.
-
Parallelized Droplet Vitrification Enables Single-Run Vitrification of the Whole Rat Liver Hepatocyte Yield.bioRxiv [Preprint]. 2024 Jul 17:2024.07.14.603471. doi: 10.1101/2024.07.14.603471. bioRxiv. 2024. Update in: ACS Appl Mater Interfaces. 2025 Mar 19;17(11):16507-16519. doi: 10.1021/acsami.4c19419. PMID: 39071342 Free PMC article. Updated. Preprint.
-
Overcoming ice: cutting-edge materials and advanced strategies for effective cryopreservation of biosample.J Nanobiotechnology. 2025 Mar 7;23(1):187. doi: 10.1186/s12951-025-03265-6. J Nanobiotechnology. 2025. PMID: 40050919 Free PMC article. Review.
-
Ice formation and its elimination in cryopreservation of oocytes.Res Sq [Preprint]. 2024 May 23:rs.3.rs-4144933. doi: 10.21203/rs.3.rs-4144933/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. PMID: 38826214 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

