A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness
- PMID: 36224245
- PMCID: PMC9555685
- DOI: 10.1038/s41467-022-33706-x
A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness
Abstract
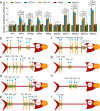
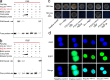
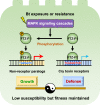
Maintaining fitness during pathogen infection is vital for host survival as an excessive response can be as detrimental as the infection itself. Fitness costs are frequently associated with insect hosts countering the toxic effect of the entomopathogenic bacterium Bacillus thuringiensis (Bt), which delay the evolution of resistance to this pathogen. The insect pest Plutella xylostella has evolved a mechanism to resist Bt toxins without incurring significant fitness costs. Here, we reveal that non-phosphorylated and phosphorylated forms of a MAPK-modulated transcription factor fushi tarazu factor 1 (FTZ-F1) can respectively orchestrate down-regulation of Bt Cry1Ac toxin receptors and up-regulation of non-receptor paralogs via two distinct binding sites, thereby presenting Bt toxin resistance without growth penalty. Our findings reveal how host organisms can co-opt a master molecular switch to overcome pathogen invasion with low cost, and contribute to understanding the underlying mechanism of growth-defense tradeoffs during host-pathogen interactions in P. xylostella.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
MAPK-dependent hormonal signaling plasticity contributes to overcoming Bacillus thuringiensis toxin action in an insect host.Nat Commun. 2020 Jun 12;11(1):3003. doi: 10.1038/s41467-020-16608-8. Nat Commun. 2020. PMID: 32532972 Free PMC article.
-
MAPK-Activated Transcription Factor PxJun Suppresses PxABCB1 Expression and Confers Resistance to Bacillus thuringiensis Cry1Ac Toxin in Plutella xylostella (L.).Appl Environ Microbiol. 2021 Jun 11;87(13):e0046621. doi: 10.1128/AEM.00466-21. Epub 2021 Jun 11. Appl Environ Microbiol. 2021. PMID: 33893113 Free PMC article.
-
MAP4K4 controlled transcription factor POUM1 regulates PxABCG1 expression influencing Cry1Ac resistance in Plutella xylostella (L.).Pestic Biochem Physiol. 2022 Mar;182:105053. doi: 10.1016/j.pestbp.2022.105053. Epub 2022 Feb 7. Pestic Biochem Physiol. 2022. PMID: 35249643
-
[Advances in receptor-mediated resistance mechanisms of Lepidopteran insects to Bacillus thuringiensis toxin].Sheng Wu Gong Cheng Xue Bao. 2022 May 25;38(5):1809-1823. doi: 10.13345/j.cjb.210834. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 35611730 Review. Chinese.
-
Early detection of field-evolved resistance to Bt cotton in China: cotton bollworm and pink bollworm.J Invertebr Pathol. 2012 Jul;110(3):301-6. doi: 10.1016/j.jip.2012.04.008. Epub 2012 Apr 16. J Invertebr Pathol. 2012. PMID: 22537835 Review.
Cited by
-
Knockdown of an ATP-binding cassette transporter in resistant western corn rootworm larvae partially reverses resistance to eCry3.1Ab protein.Sci Rep. 2024 Dec 28;14(1):31508. doi: 10.1038/s41598-024-83135-7. Sci Rep. 2024. PMID: 39733129 Free PMC article.
-
The recombinant jasmonate ZIM-domain (JAZ) protein functions as a novel insecticidal protein against Lepidopteran pests.Sci China Life Sci. 2024 Aug;67(8):1777-1778. doi: 10.1007/s11427-024-2633-5. Epub 2024 Jun 3. Sci China Life Sci. 2024. PMID: 38836957 No abstract available.
-
RNA m6 A Methylation Suppresses Insect Juvenile Hormone Degradation to Minimize Fitness Costs in Response to A Pathogenic Attack.Adv Sci (Weinh). 2024 Feb;11(6):e2307650. doi: 10.1002/advs.202307650. Epub 2023 Dec 12. Adv Sci (Weinh). 2024. PMID: 38087901 Free PMC article.
-
CRISPR/Cas Technology in Insect Insecticide Resistance.Insects. 2025 Mar 26;16(4):345. doi: 10.3390/insects16040345. Insects. 2025. PMID: 40332816 Free PMC article. Review.
-
Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: role of transcriptional and post-transcriptional events.Front Mol Biosci. 2023 Sep 6;10:1257859. doi: 10.3389/fmolb.2023.1257859. eCollection 2023. Front Mol Biosci. 2023. PMID: 37745689 Free PMC article. Review.
References
-
- Bisen, P. S., Debnath, M. & Prasad, G. B. Microbes: Concepts and Applications (John Wiley & Sons, 2012).
-
- Bertrand, J. C. et al. Environmental Microbiology: Fundamentals and Applications: Microbial Ecology (Springer, 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

