Deletion of Crtc1 leads to hippocampal neuroenergetic impairments associated with depressive-like behavior
- PMID: 36224260
- PMCID: PMC9734042
- DOI: 10.1038/s41380-022-01791-5
Deletion of Crtc1 leads to hippocampal neuroenergetic impairments associated with depressive-like behavior
Abstract
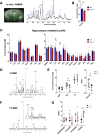
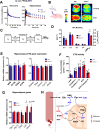
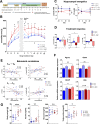
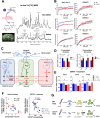
Mood disorders (MD) are a major burden on society as their biology remains poorly understood, challenging both diagnosis and therapy. Among many observed biological dysfunctions, homeostatic dysregulation, such as metabolic syndrome (MeS), shows considerable comorbidity with MD. Recently, CREB-regulated transcription coactivator 1 (CRTC1), a regulator of brain metabolism, was proposed as a promising factor to understand this relationship. Searching for imaging biomarkers and associating them with pathophysiological mechanisms using preclinical models can provide significant insight into these complex psychiatric diseases and help the development of personalized healthcare. Here, we used neuroimaging technologies to show that deletion of Crtc1 in mice leads to an imaging fingerprint of hippocampal metabolic impairment related to depressive-like behavior. By identifying a deficiency in hippocampal glucose metabolism as the underlying molecular/physiological origin of the markers, we could assign an energy-boosting mood-stabilizing treatment, ebselen, which rescued behavior and neuroimaging markers. Finally, our results point toward the GABAergic system as a potential therapeutic target for behavioral dysfunctions related to metabolic disorders. This study provides new insights on Crtc1's and MeS's relationship to MD and establishes depression-related markers with clinical potential.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
New Insights Into the Pivotal Role of CREB-Regulated Transcription Coactivator 1 in Depression and Comorbid Obesity.Front Mol Neurosci. 2022 Feb 15;15:810641. doi: 10.3389/fnmol.2022.810641. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35242012 Free PMC article. Review.
-
Hippocampal Salt-Inducible Kinase 2 Plays a Role in Depression via the CREB-Regulated Transcription Coactivator 1-cAMP Response Element Binding-Brain-Derived Neurotrophic Factor Pathway.Biol Psychiatry. 2019 Apr 15;85(8):650-666. doi: 10.1016/j.biopsych.2018.10.004. Epub 2018 Oct 18. Biol Psychiatry. 2019. PMID: 30503507
-
Gender-specific alteration of energy balance and circadian locomotor activity in the Crtc1 knockout mouse model of depression.Transl Psychiatry. 2017 Dec 8;7(12):1269. doi: 10.1038/s41398-017-0023-4. Transl Psychiatry. 2017. PMID: 29217834 Free PMC article.
-
Downregulation of CRTC1 Is Involved in CUMS-Induced Depression-Like Behavior in the Hippocampus and Its RNA Sequencing Analysis.Mol Neurobiol. 2022 Jul;59(7):4405-4418. doi: 10.1007/s12035-022-02787-6. Epub 2022 May 13. Mol Neurobiol. 2022. PMID: 35556215
-
Adeno-associated virus-mediated over-expression of CREB-regulated transcription coactivator 1 in the hippocampal dentate gyrus ameliorates lipopolysaccharide-induced depression-like behaviour in mice.J Neurochem. 2019 Apr;149(1):111-125. doi: 10.1111/jnc.14670. Epub 2019 Mar 5. J Neurochem. 2019. PMID: 30697736
Cited by
-
Amphiregulin normalizes altered circuit connectivity for social dominance of the CRTC3 knockout mouse.Mol Psychiatry. 2023 Nov;28(11):4655-4665. doi: 10.1038/s41380-023-02258-x. Epub 2023 Sep 20. Mol Psychiatry. 2023. PMID: 37730843 Free PMC article.
-
New Insights Into the Pivotal Role of CREB-Regulated Transcription Coactivator 1 in Depression and Comorbid Obesity.Front Mol Neurosci. 2022 Feb 15;15:810641. doi: 10.3389/fnmol.2022.810641. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35242012 Free PMC article. Review.
-
Quercetin reshapes gut microbiota homeostasis and modulates brain metabolic profile to regulate depression-like behaviors induced by CUMS in rats.Front Pharmacol. 2024 Mar 26;15:1362464. doi: 10.3389/fphar.2024.1362464. eCollection 2024. Front Pharmacol. 2024. PMID: 38595919 Free PMC article.
-
Excitation-transcription coupling, neuronal gene expression and synaptic plasticity.Nat Rev Neurosci. 2023 Nov;24(11):672-692. doi: 10.1038/s41583-023-00742-5. Epub 2023 Sep 29. Nat Rev Neurosci. 2023. PMID: 37773070 Free PMC article. Review.
-
High-intensity training on CREB activation for improving brain health: a narrative review of possible molecular talks.Front Endocrinol (Lausanne). 2025 Jan 20;15:1498495. doi: 10.3389/fendo.2024.1498495. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39902166 Free PMC article. Review.
References
-
- Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. - PubMed
-
- Mathers C, Fat DM, Boerma JT The global burden of disease: 2004 update. Geneva: World Health Organization; 2008.
-
- Lenzenweger MF. Endophenotype, intermediate phenotype, biomarker: definitions, concept comparisons, clarifications. Depress Anxiety. 2013;30:185–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

