Ultrasensitive colorimetric detection of fluoride and arsenate in water and mammalian cells using recyclable metal oxacalixarene probe: a lateral flow assay
- PMID: 36224315
- PMCID: PMC9556598
- DOI: 10.1038/s41598-022-21407-w
Ultrasensitive colorimetric detection of fluoride and arsenate in water and mammalian cells using recyclable metal oxacalixarene probe: a lateral flow assay
Abstract
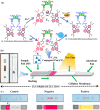
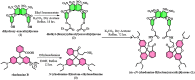
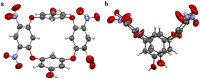
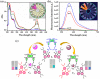
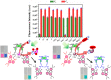
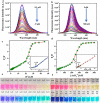
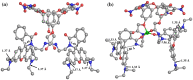
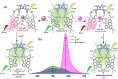
Globally 3 billion people are consuming water with moderately high concentrations of fluoride and arsenic. The development of a simple point of care (PoC) device or home device for the detection of fluoride/arsenic ensures safety before consuming water. Till date, lateral flow assay (LFA) based PoC devices can detect nucleic acids, viruses and diseases. An aluminium complex of rhodamine B functionalized oxacalix[4]arene (L) was designed to execute the LFA-based PoC device. Initially, Al3+ and Fe3+ ions were involved in complexation with the rhodamine B functionalized oxacalix[4]arene (L), resulting C1 (L-Al3+) and C2 (L-Fe3+) complexes respectively. The receptor L, as well as the probes (C1, C2), were characterized thoroughly using mass spectroscopy, FTIR, NMR, and EA. C1 and C2 were further utilized as recyclable probes for the detection of aqueous fluoride (21 ppb) and arsenate (1.92 ppb) respectively. The computational calculation indicates that upon complexation, the spirolactam ring opening at the rhodamine B site leads to optoelectronic changes. The consistency of LFA-based portable sensing device has been tested with water samples, synthetic fluoride standards and dental care products like toothpaste and mouthwash with concentrations ≥ 3 ppm. Moreover, fixed cell imaging experiments were performed to ascertain the in-vitro sensing phenomena.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Colorimetric detection of anions in aqueous media using N-monosubstituted diaminomaleonitrile-based azo-azomethine receptors: real-life applications.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Mar 15;139:405-12. doi: 10.1016/j.saa.2014.12.088. Epub 2014 Dec 25. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25576937
-
Rhodamine based dual probes for selective detection of mercury and fluoride ions in water using two mutually independent sensing pathways.Analyst. 2014 May 21;139(10):2370-8. doi: 10.1039/c3an02020g. Analyst. 2014. PMID: 24669370
-
Fluoride ions detection in aqueous media by unprecedented ring opening of fluorescein dye: A novel multimodal sensor for fluoride ions and its utilization in live cell imaging.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Feb 15;287(Pt 1):122001. doi: 10.1016/j.saa.2022.122001. Epub 2022 Oct 19. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 36334417
-
Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives.Sensors (Basel). 2024 Jun 15;24(12):3889. doi: 10.3390/s24123889. Sensors (Basel). 2024. PMID: 38931673 Free PMC article. Review.
-
Fluorides for preventing early tooth decay (demineralised lesions) during fixed brace treatment.Cochrane Database Syst Rev. 2019 Nov 17;2019(11):CD003809. doi: 10.1002/14651858.CD003809.pub4. Cochrane Database Syst Rev. 2019. PMID: 31742669 Free PMC article.
Cited by
-
Fluorescence-Based Portable Assays for Detection of Biological and Chemical Analytes.Sensors (Basel). 2023 May 25;23(11):5053. doi: 10.3390/s23115053. Sensors (Basel). 2023. PMID: 37299780 Free PMC article. Review.
References
-
- Osseiran N, Lufadeju Y. 1 in 3 People Globally Do Not Have Access to Safe Drinking Water—UNICEF. WHO; 2019.
-
- Yamamura, S. Drinking water guidelines and standards. In United Nations Synth. Rep. Arsen. Drink. Water 18 (2001).
-
- Hagenmuller, P. Inorganic Solid Fluorides: Chemistry and Physics. (Elsevier, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

