Intralesional TLR4 agonist treatment strengthens the organ defense against colonizing cancer cells in the brain
- PMID: 36224342
- PMCID: PMC9652147
- DOI: 10.1038/s41388-022-02496-3
Intralesional TLR4 agonist treatment strengthens the organ defense against colonizing cancer cells in the brain
Abstract
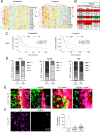
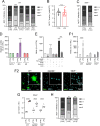
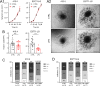
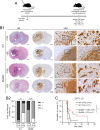
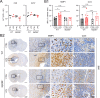
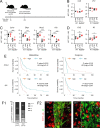
Brain metastasis in breast cancer remains difficult to treat and its incidence is increasing. Therefore, the development of new therapies is of utmost clinical relevance. Recently, toll-like receptor (TLR) 4 was correlated with IL6 expression and poor prognosis in 1 215 breast cancer primaries. In contrast, we demonstrated that TLR4 stimulation reduces microglia-assisted breast cancer cell invasion. However, the expression, prognostic value, or therapeutic potential of TLR signaling in breast cancer brain metastasis have not been investigated. We thus tested the prognostic value of various TLRs in two brain-metastasis gene sets. Furthermore, we investigated different TLR agonists, as well as MyD88 and TRIF-deficient microenvironments in organotypic brain-slice ex vivo co-cultures and in vivo colonization experiments. These experiments underline the ambiguous roles of TLR4, its adapter MyD88, and the target nitric oxide (NO) during brain colonization. Moreover, analysis of the gene expression datasets of breast cancer brain metastasis patients revealed associations of TLR1 and IL6 with poor overall survival. Finally, our finding that a single LPS application at the onset of colonization shapes the later microglia/macrophage reaction at the macro-metastasis brain-parenchyma interface (MMPI) and reduces metastatic infiltration into the brain parenchyma may prove useful in immunotherapeutic considerations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The IRAK4 scaffold integrates TLR4-driven TRIF and MYD88 signaling pathways.Cell Rep. 2022 Aug 16;40(7):111225. doi: 10.1016/j.celrep.2022.111225. Cell Rep. 2022. PMID: 35977521 Free PMC article.
-
Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages.J Immunol. 2004 Sep 1;173(5):3320-8. doi: 10.4049/jimmunol.173.5.3320. J Immunol. 2004. PMID: 15322195
-
TLR4/MyD88 signaling determines the metastatic potential of breast cancer cells.Mol Med Rep. 2018 Sep;18(3):3411-3420. doi: 10.3892/mmr.2018.9326. Epub 2018 Jul 26. Mol Med Rep. 2018. PMID: 30066873 Free PMC article.
-
Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling.Dis Model Mech. 2010 Jan-Feb;3(1-2):92-103. doi: 10.1242/dmm.003533. Epub 2009 Dec 28. Dis Model Mech. 2010. PMID: 20038715
-
The role of Toll-like receptor 4 signaling pathway in ovarian, cervical, and endometrial cancers.Life Sci. 2020 Apr 15;247:117435. doi: 10.1016/j.lfs.2020.117435. Epub 2020 Feb 17. Life Sci. 2020. PMID: 32081661 Review.
Cited by
-
Current preclinical models of brain metastasis.Clin Exp Metastasis. 2024 Dec 19;42(1):5. doi: 10.1007/s10585-024-10318-x. Clin Exp Metastasis. 2024. PMID: 39694975 Review.
-
ENO1 Promotes OSCC Migration and Invasion by Orchestrating IL-6 Secretion from Macrophages via a Positive Feedback Loop.Int J Mol Sci. 2023 Jan 1;24(1):737. doi: 10.3390/ijms24010737. Int J Mol Sci. 2023. PMID: 36614179 Free PMC article.
-
Structural insight into TLR4/MD-2 activation by synthetic LPS mimetics with distinct binding modes.Nat Commun. 2025 May 5;16(1):4164. doi: 10.1038/s41467-025-59550-3. Nat Commun. 2025. PMID: 40325026 Free PMC article.
-
The Role of the Toll-like Receptor 2 and the cGAS-STING Pathways in Breast Cancer: Friends or Foes?Int J Mol Sci. 2023 Dec 29;25(1):456. doi: 10.3390/ijms25010456. Int J Mol Sci. 2023. PMID: 38203626 Free PMC article. Review.
-
Relationship between GTP binding protein RAB10, toll-like receptor 4, and nuclear factor kappa-B and prognosis in patients with breast cancer.Sci Rep. 2024 Oct 7;14(1):23287. doi: 10.1038/s41598-024-74501-6. Sci Rep. 2024. PMID: 39375417 Free PMC article.
References
-
- Kudo Y, Haymaker C, Zhang J, Reuben A, Duose DY, Fujimoto J, et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2019;30:1521–30. doi: 10.1093/annonc/mdz207. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

