Roles of RIPK3 in necroptosis, cell signaling, and disease
- PMID: 36224345
- PMCID: PMC9636380
- DOI: 10.1038/s12276-022-00868-z
Roles of RIPK3 in necroptosis, cell signaling, and disease
Abstract
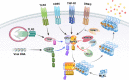
Receptor-interacting protein kinase-3 (RIPK3, or RIP3) is an essential protein in the "programmed" and "regulated" cell death pathway called necroptosis. Necroptosis is activated by the death receptor ligands and pattern recognition receptors of the innate immune system, and the findings of many reports have suggested that necroptosis is highly significant in health and human disease. This significance is largely because necroptosis is distinguished from other modes of cell death, especially apoptosis, in that it is highly proinflammatory given that cell membrane integrity is lost, triggering the activation of the immune system and inflammation. Here, we discuss the roles of RIPK3 in cell signaling, along with its role in necroptosis and various pathways that trigger RIPK3 activation and cell death. Lastly, we consider pathological situations in which RIPK3/necroptosis may play a role.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
RIPK3 in necroptosis and cancer.Mol Cells. 2025 May;48(5):100199. doi: 10.1016/j.mocell.2025.100199. Epub 2025 Feb 24. Mol Cells. 2025. PMID: 40010643 Free PMC article. Review.
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
Mechanisms of TNF-independent RIPK3-mediated cell death.Biochem J. 2022 Oct 14;479(19):2049-2062. doi: 10.1042/BCJ20210724. Biochem J. 2022. PMID: 36240069 Free PMC article. Review.
-
Spatiotemporal Control of Inflammatory Lytic Cell Death Through Optogenetic Induction of RIPK3 Oligomerization.J Mol Biol. 2024 Jul 1;436(13):168628. doi: 10.1016/j.jmb.2024.168628. Epub 2024 May 24. J Mol Biol. 2024. PMID: 38797430 Free PMC article.
-
High mobility group box 1 enables bacterial lipids to trigger receptor-interacting protein kinase 3 (RIPK3)-mediated necroptosis and apoptosis in mice.J Biol Chem. 2019 May 31;294(22):8872-8884. doi: 10.1074/jbc.RA118.007040. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000631 Free PMC article.
Cited by
-
Mitochondrial alterations and signatures in hepatocellular carcinoma.Cancer Metastasis Rev. 2025 Feb 18;44(1):34. doi: 10.1007/s10555-025-10251-9. Cancer Metastasis Rev. 2025. PMID: 39966277 Free PMC article. Review.
-
Cell death pathways: molecular mechanisms and therapeutic targets for cancer.MedComm (2020). 2024 Sep 4;5(9):e693. doi: 10.1002/mco2.693. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39239068 Free PMC article. Review.
-
The Dual Role of Necroptosis in Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2023 Aug 10;24(16):12633. doi: 10.3390/ijms241612633. Int J Mol Sci. 2023. PMID: 37628814 Free PMC article. Review.
-
TRIF-dependent signaling and its role in liver diseases.Front Cell Dev Biol. 2024 Apr 17;12:1370042. doi: 10.3389/fcell.2024.1370042. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38694821 Free PMC article. Review.
-
Susceptibility and Permissivity of Zebrafish (Danio rerio) Larvae to Cypriniviruses.Viruses. 2023 Mar 17;15(3):768. doi: 10.3390/v15030768. Viruses. 2023. PMID: 36992477 Free PMC article.
References
-
- Verma I. 2002 Nobel Prize for physiology and medicine. Mol. Ther. 2002;6:698. - PubMed
-
- Sun X, et al. RIP3, a novel apoptosis-inducing kinase. J. Biol. Chem. 1999;274:16871–16875. - PubMed
-
- Yu PW, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr. Biol. 1999;9:539–542. - PubMed
-
- Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

