Structure of the OMEGA nickase IsrB in complex with ωRNA and target DNA
- PMID: 36224386
- PMCID: PMC9581776
- DOI: 10.1038/s41586-022-05324-6
Structure of the OMEGA nickase IsrB in complex with ωRNA and target DNA
Abstract
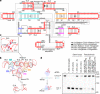
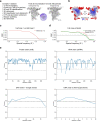
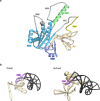
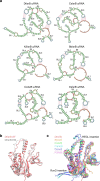
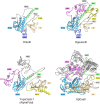
RNA-guided systems, such as CRISPR-Cas, combine programmable substrate recognition with enzymatic function, a combination that has been used advantageously to develop powerful molecular technologies1,2. Structural studies of these systems have illuminated how the RNA and protein jointly recognize and cleave their substrates, guiding rational engineering for further technology development3. Recent work identified a new class of RNA-guided systems, termed OMEGA, which include IscB, the likely ancestor of Cas9, and the nickase IsrB, a homologue of IscB lacking the HNH nuclease domain4. IsrB consists of only around 350 amino acids, but its small size is counterbalanced by a relatively large RNA guide (roughly 300-nt ωRNA). Here, we report the cryogenic-electron microscopy structure of Desulfovirgula thermocuniculi IsrB (DtIsrB) in complex with its cognate ωRNA and a target DNA. We find the overall structure of the IsrB protein shares a common scaffold with Cas9. In contrast to Cas9, however, which uses a recognition (REC) lobe to facilitate target selection, IsrB relies on its ωRNA, part of which forms an intricate ternary structure positioned analogously to REC. Structural analyses of IsrB and its ωRNA as well as comparisons to other RNA-guided systems highlight the functional interplay between protein and RNA, advancing our understanding of the biology and evolution of these diverse systems.
© 2022. The Author(s).
Conflict of interest statement
F.Z. is a scientific adviser and cofounder of Editas Medicine, Beam Therapeutics, Pairwise Plants, Arbor Biotechnologies and Proof Diagnostics.
Figures









References
-
- Zhang F. Development of CRISPR–Cas systems for genome editing and beyond. Q. Rev. Biophys. 2019;52:e6–e6. doi: 10.1017/S0033583519000052. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

