Maturation and circuit integration of transplanted human cortical organoids
- PMID: 36224417
- PMCID: PMC9556304
- DOI: 10.1038/s41586-022-05277-w
Maturation and circuit integration of transplanted human cortical organoids
Abstract
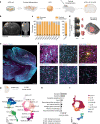
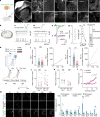
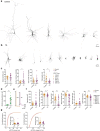
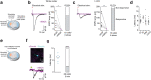
Self-organizing neural organoids represent a promising in vitro platform with which to model human development and disease1-5. However, organoids lack the connectivity that exists in vivo, which limits maturation and makes integration with other circuits that control behaviour impossible. Here we show that human stem cell-derived cortical organoids transplanted into the somatosensory cortex of newborn athymic rats develop mature cell types that integrate into sensory and motivation-related circuits. MRI reveals post-transplantation organoid growth across multiple stem cell lines and animals, whereas single-nucleus profiling shows progression of corticogenesis and the emergence of activity-dependent transcriptional programs. Indeed, transplanted cortical neurons display more complex morphological, synaptic and intrinsic membrane properties than their in vitro counterparts, which enables the discovery of defects in neurons derived from individuals with Timothy syndrome. Anatomical and functional tracings show that transplanted organoids receive thalamocortical and corticocortical inputs, and in vivo recordings of neural activity demonstrate that these inputs can produce sensory responses in human cells. Finally, cortical organoids extend axons throughout the rat brain and their optogenetic activation can drive reward-seeking behaviour. Thus, transplanted human cortical neurons mature and engage host circuits that control behaviour. We anticipate that this approach will be useful for detecting circuit-level phenotypes in patient-derived cells that cannot otherwise be uncovered.
© 2022. The Author(s).
Conflict of interest statement
Stanford University holds patents for the generation of cortical organoids/spheroids (listing S.P.P. as an inventor) and a provisional patent application for transplantation of organoids (listing S.P.P., O.R., F.G., K.D. and K.W.K. as inventors).
Figures










Comment in
-
Human brain cells implanted in rats prompt excitement - and concern.Nature. 2022 Oct;610(7932):427-428. doi: 10.1038/d41586-022-03238-x. Nature. 2022. PMID: 36224372 No abstract available.
-
Studying human neural function in vivo at the cellular level: Chasing chimeras?Cell. 2022 Dec 22;185(26):4869-4872. doi: 10.1016/j.cell.2022.11.020. Cell. 2022. PMID: 36563661
Similar articles
-
Host circuit engagement of human cortical organoids transplanted in rodents.Nat Protoc. 2024 Dec;19(12):3542-3567. doi: 10.1038/s41596-024-01029-4. Epub 2024 Jul 29. Nat Protoc. 2024. PMID: 39075308
-
Cerebral Cortex Generated from Pluripotent Stem Cells to Model Corticogenesis and Rebuild Cortical Circuits: In Vitro Veritas?Stem Cells Dev. 2019 Mar 15;28(6):361-369. doi: 10.1089/scd.2018.0233. Epub 2019 Feb 20. Stem Cells Dev. 2019. PMID: 30661489 Review.
-
Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons.Nature. 2014 Jul 24;511(7510):471-4. doi: 10.1038/nature13390. Epub 2014 May 14. Nature. 2014. PMID: 24828045
-
Modeling brain disorders using transplanted organoids: Beyond the short circuit.Cell Stem Cell. 2022 Dec 1;29(12):1617-1618. doi: 10.1016/j.stem.2022.11.002. Cell Stem Cell. 2022. PMID: 36459963
-
Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors.Brain Res. 2013 May 20;1511:73-92. doi: 10.1016/j.brainres.2012.11.001. Epub 2012 Nov 8. Brain Res. 2013. PMID: 23142759 Free PMC article. Review.
Cited by
-
Functional genomics and systems biology in human neuroscience.Nature. 2023 Nov;623(7986):274-282. doi: 10.1038/s41586-023-06686-1. Epub 2023 Nov 8. Nature. 2023. PMID: 37938705 Free PMC article. Review.
-
Myelin organoids for the study of Alzheimer's disease.Front Neurosci. 2023 Oct 24;17:1283742. doi: 10.3389/fnins.2023.1283742. eCollection 2023. Front Neurosci. 2023. PMID: 37942133 Free PMC article. No abstract available.
-
Functional Neural Networks in Human Brain Organoids.BME Front. 2024 Sep 23;5:0065. doi: 10.34133/bmef.0065. eCollection 2024. BME Front. 2024. PMID: 39314749 Free PMC article. Review.
-
Functional maturation of human iPSC-derived pyramidal neurons in vivo is dependent on proximity with the host tissue.Front Cell Neurosci. 2023 Nov 23;17:1259712. doi: 10.3389/fncel.2023.1259712. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38077953 Free PMC article.
-
Cellular reprogramming as a tool to model human aging in a dish.Nat Commun. 2024 Feb 28;15(1):1816. doi: 10.1038/s41467-024-46004-5. Nat Commun. 2024. PMID: 38418829 Free PMC article. Review.
References
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

