Efficacy and Safety of Pregabalin Prolonged Release-Etoricoxib Combination Compared to Etoricoxib for Chronic Low Back Pain: Phase 3, Randomized Study
- PMID: 36224489
- PMCID: PMC9633919
- DOI: 10.1007/s40122-022-00437-2
Efficacy and Safety of Pregabalin Prolonged Release-Etoricoxib Combination Compared to Etoricoxib for Chronic Low Back Pain: Phase 3, Randomized Study
Abstract
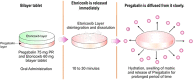
Introduction: Currently available treatments for chronic lower back pain (CLBP) do not adequately address both nociceptive and neuropathic components of pain. We evaluated efficacy and safety of fixed-dose combination (FDC) of low-dose pregabalin prolonged release 75 mg-etoricoxib 60 mg to address both pain components.
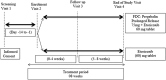
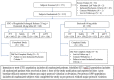
Methods: This randomized phase 3 trial conducted at 12 centres across India evaluated efficacy (based on mean change in numeric rating scale [NRS], Roland-Morris disability questionnaire [RDQ], visual analogue scale [VAS], patient global impression of improvement [PGI-I], clinical global impression of improvement [CGI-I] and rescue medication consumption) and safety of FDC in comparison to etoricoxib alone in adult patients with CLBP. Treatment duration was 8 weeks.
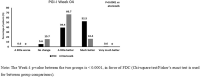
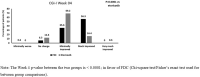
Results: Of the 371 patients screened, 319 were randomized and considered for efficacy and safety analysis. Both treatment groups had no significant difference in terms of demography and baseline disease characteristics. Significantly better outcomes with FDC compared to etoricoxib were observed at week 4 onwards. At week 8, both groups showed significant reduction in mean NRS score from baseline (- 4.00 ± 1.65 in FDC; - 2.92 ± 1.59 in etoricoxib) with mean NRS score being significantly less in the FDC group compared to etoricoxib group (3.26 ± 1.56 vs 4.31 ± 1.56; p < 0.0001). The FDC was more effective than etoricoxib in terms of significantly greater reduction in RDQ score (- 9.28 ± 4.48 vs - 6.78 ± 4.34; p < 0.0001) and VAS score (- 37.66 ± 18.7 vs - 28.50 ± 16.31; p < 0.0001) at week 8. The FDC was also better in terms of significantly more patients reporting their condition as 'very much better' (36.9% vs 5.0%; p < 0.0001) and clinicians reporting patient's condition as 'very much improved' (36.3% vs 5.7%; p < 0.0001). Overall, study medications were well tolerated.
Conclusion: FDC of pregabalin and etoricoxib provided significant benefits in reducing pain and improving functional status compared with etoricoxib alone in patients with CLBP. Pregabalin prolonged release-etoricoxib FDC could be one of the treatment options for early and sustained pain relief and improvement in quality-of-life in treating CLBP as it addresses both neuropathic and nociceptive components of pain.
Trial registration: CTRI/2018/10/015886.
Keywords: Back pain; Drug combination; Etoricoxib; Neuropathic pain; Pregabalin.
Plain language summary
Low back pain is one of the most common causes of loss of productivity worldwide. About 60% of Indians suffer from low back pain at some point. Low back pain that persists for more than 3 months is classified as chronic low back pain which mostly includes both nociceptive and neuropathic components. Monotherapies, if prescribed, are not completely effective, as they generally only target either nociceptive or neuropathic components of pain. Multiple drugs are usually needed at multiple times a day, at higher doses for optimal effectiveness, and in most cases they have significant side effects if taken over prolonged periods and also add to the pill burden. To minimize treatment-associated adverse effects, and to increase treatment compliance, while addressing both the components of pain, we developed a fixed-dose combination of low-dose pregabalin prolonged release and etoricoxib. A phase 3 trial was designed to assess the efficacy and safety of the fixed-dose combination in comparison with etoricoxib alone in treating chronic low back pain. The combination demonstrated statistically and clinically significant improvement in patient-reported outcomes—pain, functionality and quality of life—as early as 4 weeks after starting the medication. No severe or serious adverse effects were reported. Thus, the combination of low-dose pregabalin prolonged release and etoricoxib could provide an option for optimal management of chronic low back pain. This would provide multiple benefits, such as addressing both nociceptive and neuropathic components of chronic low back pain, reducing drug-related adverse effects because of low dose, reducing pill burden and thereby increasing drug compliance.
© 2022. The Author(s).
Figures






Similar articles
-
Fixed dose combination of low dose pregabalin and duloxetine, or pregabalin monotherapy for neuropathic pain: A double-blind, randomized, parallel-group study.F1000Res. 2023 Mar 30;12:353. doi: 10.12688/f1000research.130345.1. eCollection 2023. F1000Res. 2023. PMID: 38618021 Free PMC article. Clinical Trial.
-
Efficacy of etoricoxib 60 mg/day and diclofenac 150 mg/day in reduction of pain and disability in patients with chronic low back pain: results of a 4-week, multinational, randomized, double-blind study.Curr Med Res Opin. 2005 Dec;21(12):2037-49. doi: 10.1185/030079905X75069. Curr Med Res Opin. 2005. PMID: 16368055 Clinical Trial.
-
Multimodal Analgesia Approach in Acute Low Back Pain Management: A Phase III Study of a Novel Analgesic Combination of Etoricoxib/Tramadol.Pain Ther. 2024 Dec;13(6):1511-1528. doi: 10.1007/s40122-024-00653-y. Epub 2024 Sep 10. Pain Ther. 2024. PMID: 39256291 Free PMC article.
-
A Critical Review on Analytical Methods for Recently Approved FDC Drugs: Pregabalin and Etoricoxib.Crit Rev Anal Chem. 2022;52(5):1048-1068. doi: 10.1080/10408347.2020.1855411. Epub 2020 Dec 13. Crit Rev Anal Chem. 2022. PMID: 33307732 Review.
-
Interventions for treating neuropathic pain in people with sickle cell disease.Cochrane Database Syst Rev. 2019 Jul 5;7(7):CD012943. doi: 10.1002/14651858.CD012943.pub2. Cochrane Database Syst Rev. 2019. PMID: 31273755 Free PMC article.
Cited by
-
Combined Efficacy of Systemically Acting Diclofenac Sodium Patch and Alpha-2-Delta Calcium Channel Ligand in Chronic Low Back Pain: Subanalysis of a Phase III Study.Pain Ther. 2023 Dec;12(6):1439-1454. doi: 10.1007/s40122-023-00558-2. Epub 2023 Oct 20. Pain Ther. 2023. PMID: 37864060 Free PMC article.
-
A Meta-analysis Exploring the Efficacy of Neuropathic Pain Medication for Low Back Pain or Spine-Related Leg Pain: Is Efficacy Dependent on the Presence of Neuropathic Pain?Drugs. 2024 Dec;84(12):1603-1636. doi: 10.1007/s40265-024-02085-6. Epub 2024 Oct 26. Drugs. 2024. PMID: 39455546 Free PMC article.
References
-
- Koley S, Sandhu NK. An association of body composition components with the menopausal status of patients with low back pain in Tarn Taran, Punjab, India. J Life Sci. 2009;1(2):129–132. doi: 10.1080/09751270.2009.11885144. - DOI
LinkOut - more resources
Full Text Sources
Medical

