Development and validation of an extended Cox prognostic model for patients with ER/PR+ and HER2- breast cancer: a retrospective cohort study
- PMID: 36224558
- PMCID: PMC9555115
- DOI: 10.1186/s12957-022-02790-0
Development and validation of an extended Cox prognostic model for patients with ER/PR+ and HER2- breast cancer: a retrospective cohort study
Abstract
Background: The purpose of this study was to explore a new estrogen receptor (ER) and/or progesterone receptor (PR)+ and human epidermal growth factor receptor 2 (HER2)- breast cancer prognostic model, called the extended Cox prognostic model, for determining the cutoff values for multiple continuous prognostic factors and their interaction via the new model concept and variable selection method.
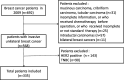
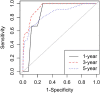
Methods: A total of 335 patients with ER/PR+ and HER2- breast cancer were enrolled for the final analysis. The primary endpoint was breast cancer-specific mortality (BCSM). Prognostic factors (histological grade, histological type, stage, T, N, lymphovascular invasion (LVI), P53, Ki67, ER, PR, and age) were included in this study. The four continuous variables (Ki67, ER, PR, and age) were partitioned into a series of binary variables that were fitted in the multivariate Cox analysis. A smoothly clipped absolute deviation (SCAD) variable selection method was used. Model performance was expressed in discrimination and calibration.
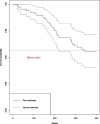
Results: We developed an extended Cox model with a time threshold of 164-week (more than 3 years) postoperation and developed a user-friendly nomogram based on our extended Cox model to facilitate clinical application. We found that the cutoff values for PR, Ki67, and age were 20%, 60%, and 41-55 years, respectively. There was an interaction between age and PR for patients aged ≥ 41 years and PR ≥ 20% at 164-week postoperation: the older the patients with ER/PR+, HER2-, and PR ≥ 20% were, the lower the survival and more likely to recur and metastasize exceeding 164 weeks (more than 3 years) after surgery.
Conclusions: Our study offers guidance on the prognosis of patients with ER/PR+ and HER2- breast cancer in China. The new concept can inform modeling and the determination of cutoff values of prognostic factors in the future.
Keywords: Breast cancer; Clinicopathological prognostic factor; Estrogen receptor; Human epidermal growth factor receptor 2; Progesterone receptor; Prognostic model.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Retrospective analysis of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), Ki67 changes and their clinical significance between primary breast cancer and metastatic tumors.PeerJ. 2024 May 15;12:e17377. doi: 10.7717/peerj.17377. eCollection 2024. PeerJ. 2024. PMID: 38766488 Free PMC article.
-
Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women.Breast J. 2013 Jan-Feb;19(1):22-30. doi: 10.1111/tbj.12059. Epub 2012 Dec 13. Breast J. 2013. PMID: 23240985
-
Prognostic values of negative estrogen or progesterone receptor expression in patients with luminal B HER2-negative breast cancer.World J Surg Oncol. 2016 Sep 13;14(1):244. doi: 10.1186/s12957-016-0999-x. World J Surg Oncol. 2016. PMID: 27619909 Free PMC article.
-
Diagnostic performance of ultrasound-based artificial intelligence for predicting key molecular markers in breast cancer: A systematic review and meta-analysis.PLoS One. 2024 May 31;19(5):e0303669. doi: 10.1371/journal.pone.0303669. eCollection 2024. PLoS One. 2024. PMID: 38820391 Free PMC article.
-
Prognostic models for breast cancer: a systematic review.BMC Cancer. 2019 Mar 14;19(1):230. doi: 10.1186/s12885-019-5442-6. BMC Cancer. 2019. PMID: 30871490 Free PMC article.
Cited by
-
Nomograms for Predicting Disease-Free Survival Based on Core Needle Biopsy and Surgical Specimens in Female Breast Cancer Patients with Non-Pathological Complete Response to Neoadjuvant Chemotherapy.J Pers Med. 2023 Jan 29;13(2):249. doi: 10.3390/jpm13020249. J Pers Med. 2023. PMID: 36836483 Free PMC article.
References
-
- Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. doi: 10.1093/annonc/mdz235. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous