Two circPPFIA1s negatively regulate liver metastasis of colon cancer via miR-155-5p/CDX1 and HuR/RAB36
- PMID: 36224588
- PMCID: PMC9555114
- DOI: 10.1186/s12943-022-01667-w
Two circPPFIA1s negatively regulate liver metastasis of colon cancer via miR-155-5p/CDX1 and HuR/RAB36
Abstract
Background: Circular RNAs (circRNAs) play a critical role in colorectal cancer (CRC) progression, including metastasis. However, the detailed molecular mechanism is not fully understood.
Methods: Differentially expressed circRNAs between primary KM12C and liver metastatic KM12L4 colon cancer cells were identified by microarray. The expression of circRNAs was measured by semi-quantitative (semi-qPCR) and real time-quantitative PCR (RT-qPCR). Metastatic potential including invasive and migratory abilities, and liver metastasis were examined by transwell assays and intrasplenic injection, respectively. CircPPFIA1-associated microRNA (miRNA) and RNA-binding protein (RBP) were screened by an antisense oligonucleotide (ASO) pulldown experiment. The effects of circPPFIA1 on target gene expression were evaluated by RT-qPCR and western blot analyses.
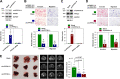
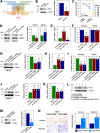
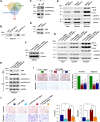
Results: By analyzing circRNA microarray data, we identified two anti-metastatic circRNAs generated from PPFIA1 with different length, which named circPPFIA1-L (long) and -S (short). They were significantly downregulated in liver metastatic KM12L4 cells compared to primary KM12C cells. The knockdown of circPPFIA1s in KM12C enhanced metastatic potential and increased liver metastasis. Conversely, overexpression of circPPFIA1s weakened metastatic potential and inhibited liver metastasis. circPPFIA1s were found to function as sponges of oncogenic miR-155-5p and Hu antigen R (HuR) by an ASO pulldown experiment. circPPFIA1s upregulated tumor-suppressing CDX1 expression and conversely downregulated oncogenic RAB36 by decoying miR-155-5p and by sequestering HuR, respectively.
Conclusion: Our findings demonstrate that circPPFIA1s inhibit the liver metastasis of CRC via the miR-155-5p/CDX1 and HuR/RAB36 pathways.
Keywords: CDX1; Colorectal cancer; HuR; Liver metastasis; RAB36; circPPFIA1; miR-155-5p.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






Similar articles
-
CircCCNB1 silencing acting as a miR-106b-5p sponge inhibited GPM6A expression to promote HCC progression by enhancing DYNC1I1 expression and activating the AKT/ERK signaling pathway.Int J Biol Sci. 2022 Jan 1;18(2):637-651. doi: 10.7150/ijbs.66915. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002514 Free PMC article.
-
Circular RNA circ0104103 inhibits colorectal cancer progression through interactions with HuR and miR-373-5p.Cancer Sci. 2023 Apr;114(4):1396-1409. doi: 10.1111/cas.15695. Epub 2023 Jan 11. Cancer Sci. 2023. PMID: 36562402 Free PMC article.
-
The CircRNA-ACAP2/Hsa-miR-21-5p/ Tiam1 Regulatory Feedback Circuit Affects the Proliferation, Migration, and Invasion of Colon Cancer SW480 Cells.Cell Physiol Biochem. 2018;49(4):1539-1550. doi: 10.1159/000493457. Epub 2018 Sep 13. Cell Physiol Biochem. 2018. PMID: 30212824
-
The circular RNA circ-ERBIN promotes growth and metastasis of colorectal cancer by miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1α translation.Mol Cancer. 2020 Nov 23;19(1):164. doi: 10.1186/s12943-020-01272-9. Mol Cancer. 2020. PMID: 33225938 Free PMC article.
-
Unraveling the Regulatory Role of HuR/microRNA Axis in Colorectal Cancer Tumorigenesis.Cancers (Basel). 2024 Sep 18;16(18):3183. doi: 10.3390/cancers16183183. Cancers (Basel). 2024. PMID: 39335155 Free PMC article. Review.
Cited by
-
CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling.Cancers (Basel). 2023 Mar 30;15(7):2068. doi: 10.3390/cancers15072068. Cancers (Basel). 2023. PMID: 37046729 Free PMC article.
-
The miR-155-5p/FBXO11 axis inhibits the progression of gastric cancer via the mTOR pathway.Transl Cancer Res. 2025 Feb 28;14(2):1375-1387. doi: 10.21037/tcr-2025-8. Epub 2025 Feb 26. Transl Cancer Res. 2025. PMID: 40104748 Free PMC article.
-
CTHRC1: a key player in colorectal cancer progression and immune evasion.Front Immunol. 2025 Mar 25;16:1579661. doi: 10.3389/fimmu.2025.1579661. eCollection 2025. Front Immunol. 2025. PMID: 40201173 Free PMC article. Review.
-
The LncRNA GABARAPL3/miR-155-5p axis modulates the proliferation and invasion of liver cancer cells by regulating GABARAPL1.Mol Biol Rep. 2025 Jun 28;52(1):650. doi: 10.1007/s11033-025-10698-7. Mol Biol Rep. 2025. PMID: 40580217 No abstract available.
-
Specific intracellular retention of circSKA3 promotes colorectal cancer metastasis by attenuating ubiquitination and degradation of SLUG.Cell Death Dis. 2023 Nov 16;14(11):750. doi: 10.1038/s41419-023-06279-w. Cell Death Dis. 2023. PMID: 37973787 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9. doi: 10.1097/01.sla.0000217629.94941.cf. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

