Immunotheranostic microbubbles (iMBs) - a modular platform for dendritic cell vaccine delivery applied to breast cancer immunotherapy
- PMID: 36224614
- PMCID: PMC9555090
- DOI: 10.1186/s13046-022-02501-3
Immunotheranostic microbubbles (iMBs) - a modular platform for dendritic cell vaccine delivery applied to breast cancer immunotherapy
Erratum in
-
Correction: Immunotheranostic microbubbles (iMBs) - a modular platform for dendritic cell vaccine delivery applied to breast cancer immunotherapy.J Exp Clin Cancer Res. 2022 Dec 24;41(1):357. doi: 10.1186/s13046-022-02577-x. J Exp Clin Cancer Res. 2022. PMID: 36564801 Free PMC article. No abstract available.
Abstract
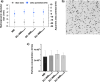
Background: Therapeutic strategies engaging the immune system against malignant cells have revolutionized the field of oncology. Proficiency of dendritic cells (DCs) for antigen presentation and immune response has spurred interest on DC-based vaccines for anti-cancer therapy. However, despite favorable safety profiles in patients, current DC-vaccines have not yet presented significant outcome due to technical barriers in active DC delivery, tumor progression, and immune dysfunction. To maximize the therapeutic response, we present here a unique cell-free DC-based vaccine capable of lymphoid organ targeting and eliciting T-cell-mediated anti-tumor effect.
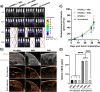
Methods: We developed this novel immunotheranostic platform using plasma membranes derived from activated DCs incorporated into ultrasound contrast microbubbles (MBs), thereby offering real-time visualization of MBs' trafficking and homing in vivo. Human PBMC-derived DCs were cultured ex vivo for controlled maturation and activation using cell membrane antigens from breast cancer cells. Following DC membrane isolation, immunotheranostic microbubbles, called DC-iMBs, were formed for triple negative breast cancer treatment in a mouse model harboring a human reconstituted immune system.
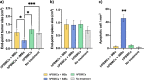
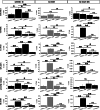
Results: Our results demonstrated that DC-iMBs can accumulate in lymphoid organs and induce anti-tumor immune response, which significantly reduced tumor growth via apoptosis while increasing survival length of the treated animals. The phenotypic changes in immune cell populations upon DC-iMBs delivery further confirmed the T-cell-mediated anti-tumor effect.
Conclusion: These early findings strongly support the potential of DC-iMBs as a novel immunotherapeutic cell-free vaccine for anti-cancer therapy.
Keywords: Breast Cancer; Dendritic cell vaccine; Immunotherapy; Microbubbles; Molecular imaging; Oncology; Ultrasound (US).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer.Mol Cancer. 2022 Feb 11;21(1):45. doi: 10.1186/s12943-022-01515-x. Mol Cancer. 2022. PMID: 35148751 Free PMC article.
-
miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy.Cancer Immunol Immunother. 2020 Jun;69(6):951-967. doi: 10.1007/s00262-020-02507-w. Epub 2020 Feb 20. Cancer Immunol Immunother. 2020. PMID: 32076794 Free PMC article.
-
Correction: Immunotheranostic microbubbles (iMBs) - a modular platform for dendritic cell vaccine delivery applied to breast cancer immunotherapy.J Exp Clin Cancer Res. 2022 Dec 24;41(1):357. doi: 10.1186/s13046-022-02577-x. J Exp Clin Cancer Res. 2022. PMID: 36564801 Free PMC article. No abstract available.
-
Ex vivo pulsed dendritic cell vaccination against cancer.Acta Pharmacol Sin. 2020 Jul;41(7):959-969. doi: 10.1038/s41401-020-0415-5. Epub 2020 May 4. Acta Pharmacol Sin. 2020. PMID: 32366940 Free PMC article. Review.
-
Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy.Biomed Pharmacother. 2023 Jun;162:114685. doi: 10.1016/j.biopha.2023.114685. Epub 2023 Apr 12. Biomed Pharmacother. 2023. PMID: 37058818 Review.
Cited by
-
Exosomal ITGB2 Mediates Immune Evasion in Triple-Negative Breast Cancer by Suppressing Dendritic Cell Activation via TLR4.Iran J Public Health. 2025 Jun;54(6):1252-1262. doi: 10.18502/ijph.v54i6.18903. Iran J Public Health. 2025. PMID: 40655508 Free PMC article.
-
Extracellular vesicles in tumor immunity: mechanisms and novel insights.Mol Cancer. 2025 Feb 14;24(1):45. doi: 10.1186/s12943-025-02233-w. Mol Cancer. 2025. PMID: 39953480 Free PMC article. Review.
-
Targeted Microbubbles for Drug, Gene, and Cell Delivery in Therapy and Immunotherapy.Pharmaceutics. 2023 May 30;15(6):1625. doi: 10.3390/pharmaceutics15061625. Pharmaceutics. 2023. PMID: 37376072 Free PMC article. Review.
-
Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ.Int J Mol Sci. 2023 Jun 8;24(12):9918. doi: 10.3390/ijms24129918. Int J Mol Sci. 2023. PMID: 37373062 Free PMC article.
-
Advances in immunotherapy and targeted therapy for advanced clear-cell renal cell carcinoma: current strategies and future directions.Front Immunol. 2025 Jul 3;16:1582887. doi: 10.3389/fimmu.2025.1582887. eCollection 2025. Front Immunol. 2025. PMID: 40677703 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

