Implantation of adipose-derived mesenchymal stem cell sheets promotes axonal regeneration and restores bladder function after spinal cord injury
- PMID: 36224621
- PMCID: PMC9558366
- DOI: 10.1186/s13287-022-03188-1
Implantation of adipose-derived mesenchymal stem cell sheets promotes axonal regeneration and restores bladder function after spinal cord injury
Abstract
Background: Cell-based therapy using adipose-derived mesenchymal stem cells (ADSCs) is a promising treatment strategy for neurogenic bladder (NB) associated with spinal cord injury (SCI). However, therapeutic efficacy is low because of inefficient cell delivery. Cell sheets improve the efficacy of cell transplantation. Therefore, this study was conducted to investigate the therapeutic efficacy of transplanting ADSC sheets into an SCI rat model and focused on the function and pathological changes of the bladder.
Methods: ADSC sheets were prepared from adipose tissue of Sprague-Dawley (SD) rats using temperature-responsive cell culture dishes. Adult female SD rats were subjected to SCI by transection at the T10 level and administered ADSC sheets or gelatin sponge (the control group). Four and 8 weeks later, in vivo cystometrograms were obtained for voiding function assessment. Rats were sacrificed and the expression of various markers was analyzed in spinal and bladder tissues.
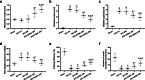
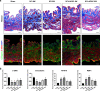
Results: The number of β-tubulin III-positive axons in the ADSC sheet transplantation group was higher than that in the control group. Conversely, expression of glial fibrillary acidic protein in the ADSC sheet transplantation group was lower than that in the control group. Cystometry showed impairment of the voiding function after SCI, which was improved after ADSC sheet transplantation with increased high-frequency oscillation activity. Furthermore, ADSC sheet transplantation prevented disruption of the bladder urothelium in SCI rats, thereby maintaining the intact barrier. Compared with fibrosis of the bladder wall in the control group, the ADSC sheet transplantation group had normal morphology of the bladder wall and reduced tissue fibrosis as shown by downregulation of type 1 collagen. ADSC sheet transplantation also resulted in strong upregulation of contractile smooth muscle cell (SMC) markers (α-smooth muscle actin and smoothelin) and downregulation of synthetic SMC markers (MYH10 and RBP1).
Conclusion: ADSC sheet transplantation significantly improved voiding function recovery in rats after SCI. ADSC sheet transplantation is a promising cell delivery and treatment option for NB related to SCI.
Keywords: Adipose-derived mesenchymal stem cells (ADSCs); Cell sheets; Cell-based therapy; Neurogenic bladder (NB); Spinal cord injury (SCI).
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures




Similar articles
-
Adipose-Derived Stem Cell Therapy in Spinal Cord Injury.Cells. 2024 Sep 9;13(17):1505. doi: 10.3390/cells13171505. Cells. 2024. PMID: 39273075 Free PMC article. Review.
-
Bi-layered Adipose Mesenchymal Cell Sheets Improve Bladder Compliance in Spinal Cord-Injured Rats.Tissue Eng Part A. 2025 May;31(9-10):409-418. doi: 10.1089/ten.TEA.2024.0115. Epub 2024 Aug 7. Tissue Eng Part A. 2025. PMID: 39041611
-
Bone marrow stromal cell sheets may promote axonal regeneration and functional recovery with suppression of glial scar formation after spinal cord transection injury in rats.J Neurosurg Spine. 2017 Mar;26(3):388-395. doi: 10.3171/2016.8.SPINE16250. Epub 2016 Nov 25. J Neurosurg Spine. 2017. PMID: 27885959
-
Porous gelatin microspheres implanted with adipose mesenchymal stromal cells promote angiogenesis via protein kinase B/endothelial nitric oxide synthase signaling pathway in bladder reconstruction.Cytotherapy. 2023 Dec;25(12):1317-1330. doi: 10.1016/j.jcyt.2023.08.005. Epub 2023 Oct 6. Cytotherapy. 2023. PMID: 37804283
-
The application of stem cell sheets for neuronal regeneration after spinal cord injury: a systematic review of pre-clinical studies.Syst Rev. 2023 Nov 30;12(1):225. doi: 10.1186/s13643-023-02390-3. Syst Rev. 2023. PMID: 38037129 Free PMC article.
Cited by
-
Hydrogel-encapsulated extracellular vesicles for the regeneration of spinal cord injury.Front Neurosci. 2023 Dec 14;17:1309172. doi: 10.3389/fnins.2023.1309172. eCollection 2023. Front Neurosci. 2023. PMID: 38156267 Free PMC article. Review.
-
Exploring the therapeutic potential of Sirt6-enriched adipose stem cell-derived exosomes in myocardial ischemia-reperfusion injury: unfolding new epigenetic frontiers.Clin Epigenetics. 2024 Jan 3;16(1):7. doi: 10.1186/s13148-023-01618-2. Clin Epigenetics. 2024. PMID: 38172884 Free PMC article.
-
Adipose-Derived Stem Cell Therapy in Spinal Cord Injury.Cells. 2024 Sep 9;13(17):1505. doi: 10.3390/cells13171505. Cells. 2024. PMID: 39273075 Free PMC article. Review.
-
Tissue Engineering and Stem Cell Therapy in Neurogenic Bladder Dysfunction: Current and Future Perspectives.Medicina (Kaunas). 2023 Aug 3;59(8):1416. doi: 10.3390/medicina59081416. Medicina (Kaunas). 2023. PMID: 37629705 Free PMC article. Review.
-
Subpial transplantation of adipose-derived stem cells alleviates paraplegia in a rat model of aortic occlusion/reperfusion-induced spinal cord infarction.Regen Ther. 2024 Aug 21;26:611-619. doi: 10.1016/j.reth.2024.08.005. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39263357 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

