Midazolam exhibits antitumour and enhances the efficiency of Anti-PD-1 immunotherapy in hepatocellular carcinoma
- PMID: 36224624
- PMCID: PMC9555186
- DOI: 10.1186/s12935-022-02735-3
Midazolam exhibits antitumour and enhances the efficiency of Anti-PD-1 immunotherapy in hepatocellular carcinoma
Abstract
Background: Midazolam (MDZ) is an anaesthetic that is widely used for anxiolysis and sedation. More recently, MDZ has also been described to be related to the outcome of various types of carcinomas. However, how MDZ influences the progression of hepatocellular carcinoma (HCC) and its effects on the biological function and tumour immune microenvironment of this type of tumour remain unknown.
Methods: The effects of MDZ on the proliferation, invasion, and migration of HCC cell lines were examined in vitro using the Cell Counting Kit 8 (CCK8), 5-ethynyl-2'-deoxyuridine (EdU), Transwell, and wound healing assays. Additionally, western blotting was employed to confirm that PD-L1 was expressed. Chromatin immunoprecipitation-seq (ChIP-seq) analysis was used to pinpoint the transcriptional regulation regions of NF-κB and programmed death-ligand 1 (PD-L1). A C57BL/6 mouse model was used to produce subcutaneous HCC tumors in order to evaluate the in vivo performance of MDZ. Mass spectrometry was also used to assess changes in the tumour immunological microenvironment following MDZ injection.
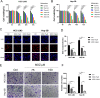
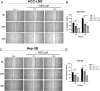
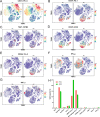
Results: The HCC-LM3 and Hep-3B cell lines' proliferation, invasion, and migration were controlled by MDZ, according to the results of the CCK8, EdU, Transwell, and wound healing assays. PD-L1 expression was shown by ChIP-seq analysis to be boosted by NF-κB, and by Western blotting analysis, it was shown that MDZ downregulated the expression of NF-κB. Additionally, in vivo tests revealed that intraperitoneal MDZ injections reduced HCC tumor development and enhanced the effectiveness of anti-PD-1 therapy. The CD45+ immune cell proportions were higher in the MDZ group than in the PBS group, according to the mass spectrometry results. Injection of MDZ resulted in a decrease in the proportions of CD4+ T cells, CD8+ T cells, natural killer (NK) cells, monocytes, Tregs, and M2 macrophages and a rise in the proportion of dendritic cells. Additionally, the concentrations of the cytokines IFN-g and TNF-a were noticeably raised whereas the concentrations of the CD8+ T-cell fatigue markers ICOS, TIGIT, and TIM3 were noticeably lowered.
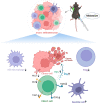
Conclusion: According to this study, MDZ inhibited the progression of HCC by inhibiting the NF-κB pathway and reducing the exhaustion of CD8+ T cells. In clinical practice, MDZ combined with anti-PD-1 therapy might contribute to synergistically improving the antitumor efficacy of HCC treatment.
Keywords: Hepatocellular carcinoma; Immune escape; MDZ; PD-1; PD-L1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Shuyu pills inhibit immune escape and enhance chemosensitization in hepatocellular carcinoma.World J Gastrointest Oncol. 2021 Nov 15;13(11):1725-1740. doi: 10.4251/wjgo.v13.i11.1725. World J Gastrointest Oncol. 2021. PMID: 34853646 Free PMC article.
-
Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade.Gut. 2019 Sep;68(9):1653-1666. doi: 10.1136/gutjnl-2019-318419. Epub 2019 Mar 22. Gut. 2019. PMID: 30902885
-
Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells.Gastroenterology. 2020 Feb;158(3):664-678.e24. doi: 10.1053/j.gastro.2019.10.025. Epub 2019 Oct 31. Gastroenterology. 2020. PMID: 31678303
-
Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review.Front Immunol. 2020 May 28;11:1037. doi: 10.3389/fimmu.2020.01037. eCollection 2020. Front Immunol. 2020. PMID: 32547550 Free PMC article. Review.
-
The immune landscape of hepatocellular carcinoma-where we are?Oncol Lett. 2022 Sep 27;24(5):410. doi: 10.3892/ol.2022.13530. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36245826 Free PMC article. Review.
Cited by
-
Remimazolam induced cytotoxicity mediated through multiple stress pathways and acted synergistically with tyrosine kinase inhibitors in hepatocellular carcinoma.Redox Rep. 2025 Dec;30(1):2475696. doi: 10.1080/13510002.2025.2475696. Epub 2025 Mar 7. Redox Rep. 2025. PMID: 40053437 Free PMC article.
-
Tumor Necrosis Factor Alpha: Implications of Anesthesia on Cancers.Cancers (Basel). 2023 Jan 25;15(3):739. doi: 10.3390/cancers15030739. Cancers (Basel). 2023. PMID: 36765695 Free PMC article. Review.
-
Hsa_circ_0007991 Promotes Immune Evasion in Hepatocellular Carcinoma via Regulation of the miR-505-3p/CANX Axis.J Hepatocell Carcinoma. 2025 Jul 7;12:1337-1351. doi: 10.2147/JHC.S513120. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40655469 Free PMC article.
-
Role of midazolam on cancer progression/survival - An updated systematic review.Indian J Anaesth. 2023 Nov;67(11):951-961. doi: 10.4103/ija.ija_731_23. Epub 2023 Nov 7. Indian J Anaesth. 2023. PMID: 38213688 Free PMC article.
-
New insights into T-cell exhaustion in liver cancer: from mechanism to therapy.J Cancer Res Clin Oncol. 2023 Oct;149(13):12543-12560. doi: 10.1007/s00432-023-05083-5. Epub 2023 Jul 9. J Cancer Res Clin Oncol. 2023. PMID: 37423958 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–249. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

