Genome editing technologies, mechanisms and improved production of therapeutic phytochemicals: Opportunities and prospects
- PMID: 36224758
- PMCID: PMC10091730
- DOI: 10.1002/bit.28260
Genome editing technologies, mechanisms and improved production of therapeutic phytochemicals: Opportunities and prospects
Abstract
Plants produce a large number of secondary metabolites, known as phytometabolites that may be employed as medicines, dyes, poisons, and insecticides in the field of medicine, agriculture, and industrial use, respectively. The rise of genome management approaches has promised a factual revolution in genetic engineering. Targeted genome editing in living entities permits the understanding of the biological systems very clearly, and also sanctions to address a wide-ranging objective in the direction of improving features of plant and their yields. The last few years have introduced a number of unique genome editing systems, including transcription activator-like effector nucleases, zinc finger nucleases, and miRNA-regulated clustered regularly interspaced short palindromic repeats/Cas9 (CRISPR/Cas9). Genome editing systems have helped in the transformation of metabolic engineering, allowing researchers to modify biosynthetic pathways of different secondary metabolites. Given the growing relevance of editing genomes in plant research, the exciting novel methods are briefly reviewed in this chapter. Also, this chapter highlights recent discoveries on the CRISPR-based modification of natural products in different medicinal plants.
Keywords: CRISPR/Cas; biosynthesis pathway; gene encoding; homozygous mutants; knockout; next-generation sequencing.
© 2022 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
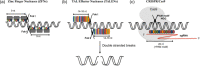

References
-
- Amitai, G. , & Sorek, R. (2016). CRISPR–Cas adaptation: Insights into the mechanism of action. Nature Reviews Microbiology, 14(2), 67–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

