Acceleration of Mesenchymal-to-Epithelial Transition (MET) during Direct Reprogramming Using Natural Compounds
- PMID: 36224763
- PMCID: PMC9668095
- DOI: 10.4014/jmb.2208.08042
Acceleration of Mesenchymal-to-Epithelial Transition (MET) during Direct Reprogramming Using Natural Compounds
Erratum in
-
Erratum to: Acceleration of Mesenchymal-to-Epithelial Transition (MET) during Direct Reprogramming Using Natural Compounds.J Microbiol Biotechnol. 2022 Dec 28;32(12):1632. doi: 10.4014/jmb.2022.3212.1632. J Microbiol Biotechnol. 2022. PMID: 36575652 Free PMC article. No abstract available.
Abstract
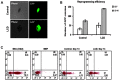
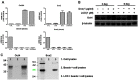
Induced pluripotent stem cells (iPSCs) can be generated from somatic cells using Oct4, Sox2, Klf4, and c-Myc (OSKM). Small molecules can enhance reprogramming. Licochalcone D (LCD), a flavonoid compound present mainly in the roots of Glycyrrhiza inflata, acts on known signaling pathways involved in transcriptional activity and signal transduction, including the PGC1-α and MAPK families. In this study, we demonstrated that LCD improved reprogramming efficiency. LCD-treated iPSCs (LCD-iPSCs) expressed pluripotency-related genes Oct4, Sox2, Nanog, and Prdm14. Moreover, LCD-iPSCs differentiated into all three germ layers in vitro and formed chimeras. The mesenchymal-to-epithelial transition (MET) is critical for somatic cell reprogramming. We found that the expression levels of mesenchymal genes (Snail2 and Twist) decreased and those of epithelial genes (DSP, Cldn3, Crb3, and Ocln) dramatically increased in OR-MEF (OG2+/+/ROSA26+/+) cells treated with LCD for 3 days, indicating that MET effectively occurred in LCD-treated OR-MEF cells. Thus, LCD enhanced the generation of iPSCs from somatic cells by promoting MET at the early stages of reprogramming.
Keywords: LCD; MET; iPSC; in vitro; natural compound; reprogramming.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures


Similar articles
-
Reactivation of Endogenous Genes and Epigenetic Remodeling Are Barriers for Generating Transgene-Free Induced Pluripotent Stem Cells in Pig.PLoS One. 2016 Jun 23;11(6):e0158046. doi: 10.1371/journal.pone.0158046. eCollection 2016. PLoS One. 2016. PMID: 27336671 Free PMC article.
-
Quick, Coordinated and Authentic Reprogramming of Ribosome Biogenesis during iPSC Reprogramming.Cells. 2020 Nov 15;9(11):2484. doi: 10.3390/cells9112484. Cells. 2020. PMID: 33203179 Free PMC article.
-
miR-524-5p of the primate-specific C19MC miRNA cluster targets TP53IPN1- and EMT-associated genes to regulate cellular reprogramming.Stem Cell Res Ther. 2017 Sep 29;8(1):214. doi: 10.1186/s13287-017-0666-3. Stem Cell Res Ther. 2017. PMID: 28962647 Free PMC article.
-
Tissue-Restricted Stem Cells as Starting Cell Source for Efficient Generation of Pluripotent Stem Cells: An Overview.Adv Exp Med Biol. 2022;1376:151-180. doi: 10.1007/5584_2021_660. Adv Exp Med Biol. 2022. PMID: 34611861 Review.
-
Understanding the roadmaps to induced pluripotency.Cell Death Dis. 2014 May 15;5(5):e1232. doi: 10.1038/cddis.2014.205. Cell Death Dis. 2014. PMID: 24832604 Free PMC article. Review.
Cited by
-
The occurrence and development of induced pluripotent stem cells.Front Genet. 2024 Apr 18;15:1389558. doi: 10.3389/fgene.2024.1389558. eCollection 2024. Front Genet. 2024. PMID: 38699229 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

