Cytoplasmic tail determines the membrane trafficking and localization of SARS-CoV-2 spike protein
- PMID: 36225258
- PMCID: PMC9548995
- DOI: 10.3389/fmolb.2022.1004036
Cytoplasmic tail determines the membrane trafficking and localization of SARS-CoV-2 spike protein
Abstract
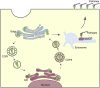
The spike (S) glycoprotein of SARS-CoV-2 mediates viral entry through associating with ACE2 on host cells. Intracellular trafficking and palmitoylation of S protein are required for its function. The short cytoplasmic tail of S protein plays a key role in the intracellular trafficking, which contains the binding site for the host trafficking proteins such as COPI, COPII and SNX27. This cytoplasmic tail also contains the palmitoylation sites of S protein. Protein palmitoylation modification of S protein could be catalyzed by a family of zinc finger DHHC domain-containing protein palmitoyltransferases (ZDHHCs). The intracellular trafficking and membrane location facilitate surface expression of S protein and assembly of progeny virions. In this review, we summarize the function of S protein cytoplasmic tail in transportation and localization. S protein relies on intracellular trafficking pathways and palmitoylation modification to facilitate the life cycle of SARS-CoV-2, meanwhile it could interfere with the host transport pathways. The interplay between S protein and intracellular trafficking proteins could partially explain the acute symptoms or Long-COVID complications in multiple organs of COVID-19 patients.
Keywords: COPI; SARS-CoV-2; SNX27; palmitoylation; spike.
Copyright © 2022 Li, Liu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- da Rosa Mesquita R., Francelino Silva Junior L. C., Santos Santana F. M., Farias de Oliveira T., Campos Alcântara R., Monteiro Arnozo G., et al. (2021). Clinical manifestations of COVID-19 in the general population: systematic review. Wien. Klin. Wochenschr. 133 (7-8), 377–382. 10.1007/s00508-020-01760-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

