PGC-1α activity and mitochondrial dysfunction in preterm infants
- PMID: 36225305
- PMCID: PMC9548560
- DOI: 10.3389/fphys.2022.997619
PGC-1α activity and mitochondrial dysfunction in preterm infants
Abstract
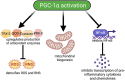
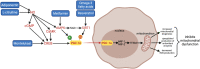
Extremely low gestational age neonates (ELGANs) are born in a relatively hyperoxic environment with weak antioxidant defenses, placing them at high risk for mitochondrial dysfunction affecting multiple organ systems including the nervous, respiratory, ocular, and gastrointestinal systems. The brain and lungs are highly affected by mitochondrial dysfunction and dysregulation in the neonate, causing white matter injury (WMI) and bronchopulmonary dysplasia (BPD), respectively. Adequate mitochondrial function is important in providing sufficient energy for organ development as it relates to alveolarization and axonal myelination and decreasing oxidative stress via reactive oxygen species (ROS) and reactive nitrogen species (RNS) detoxification. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) is a master regulator of mitochondrial biogenesis and function. Since mitochondrial dysfunction is at the root of WMI and BPD pathobiology, exploring therapies that can regulate PGC-1α activity may be beneficial. This review article describes several promising therapeutic agents that can mitigate mitochondrial dysfunction through direct and indirect activation and upregulation of the PGC-1α pathway. Metformin, resveratrol, omega 3 fatty acids, montelukast, L-citrulline, and adiponectin are promising candidates that require further pre-clinical and clinical studies to understand their efficacy in decreasing the burden of disease from WMI and BPD in preterm infants.
Keywords: PGC-1α; bronchopulmonary dysplasia; mitochondrial dysfunction; oxidative stress; reactive oxygen species; white matter injury.
Copyright © 2022 Mohammadi, Higazy and Gauda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adamia N., Virsaladze D., Charkviani N., Skhirtladze M., Khutsishvili M. (2007). Effect of metformin therapy on plasma adiponectin and leptin levels in obese and insulin resistant postmenopausal females with type 2 diabetes. Georgian Med. News 2007 (145), 52–55. - PubMed
-
- Afolayan A. J., Eis A., Teng R. J., Bakhutashvili I., Kaul S., Davis J. M., et al. (2012). Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell. Mol. Physiol. 303 (10), L870–L879. 10.1152/ajplung.00098.2012 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

