In vivo Phage Display: A promising selection strategy for the improvement of antibody targeting and drug delivery properties
- PMID: 36225354
- PMCID: PMC9549074
- DOI: 10.3389/fmicb.2022.962124
In vivo Phage Display: A promising selection strategy for the improvement of antibody targeting and drug delivery properties
Abstract
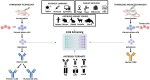
The discovery of hybridoma technology, described by Kohler and Milstein in 1975, and the resulting ability to generate monoclonal antibodies (mAbs) initiated a new era in antibody research and clinical development. However, limitations of the hybridoma technology as a routine antibody generation method in conjunction with high immunogenicity responses have led to the development of alternative approaches for the streamlined identification of most effective antibodies. Within this context, display selection technologies such as phage display, ribosome display, yeast display, bacterial display, and mammalian cell surface display have been widely promoted over the past three decades as ideal alternatives to traditional hybridoma methods. The display of antibodies on phages is probably the most widespread and powerful of these methods and, since its invention in late 1980s, significant technological advancements in the design, construction, and selection of antibody libraries have been made, and several fully human antibodies generated by phage display are currently approved or in various clinical development stages. With evolving novel disease targets and the emerging of a new generation of therapeutic antibodies, such as bispecific antibodies, antibody drug conjugates (ADCs), and chimeric antigen receptor T (CAR-T) cell therapies, it is clear that phage display is expected to continue to play a central role in antibody development. Nevertheless, for non-standard and more demanding cases aiming to generate best-in-class therapeutic antibodies against challenging targets and unmet medical needs, in vivo phage display selections by which phage libraries are directly injected into animals or humans for isolating and identifying the phages bound to specific tissues offer an advantage over conventional in vitro phage display screening procedures. Thus, in the present review, we will first summarize a general overview of the antibody therapeutic market, the different types of antibody fragments, and novel engineered variants that have already been explored. Then, we will discuss the state-of-the-art of in vivo phage display methodologies as a promising emerging selection strategy for improvement antibody targeting and drug delivery properties.
Keywords: antibody discovery; antibody engineering; antibody selection; in vivo; phage display; therapeutic antibodies.
Copyright © 2022 André, Moutinho, Dias and Aires-da-Silva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aguiar S. I., Dias J. N. R., André A. S., Silva M. L., Martins D., Carrapiço B., et al. (2021). Highly specific blood-brain barrier transmigrating single-domain antibodies selected by an in vivo phage display screening. Pharmaceutics 13:1598. doi: 10.3390/pharmaceutics13101598, PMID: - DOI - PMC - PubMed
-
- Aires da Silva F., Santa-Marta M., Freitas-Vieira A., Mascarenhas P., Barahona I., Moniz-Pereira J., et al. (2004). Camelized rabbit-derived VH single-domain intrabodies against Vif strongly neutralize HIV-1 infectivity. J. Mol. Biol. 340, 525–542. doi: 10.1016/j.jmb.2004.04.062, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

