Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction
- PMID: 36225570
- PMCID: PMC9549247
- DOI: 10.3389/fphar.2022.1012723
Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction
Abstract
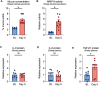
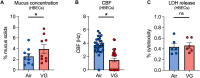
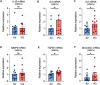
Vegetable glycerin (VG) and propylene glycol (PG) serve as delivery vehicles for nicotine and flavorings in most e-cigarette (e-cig) liquids. Here, we investigated whether VG e-cig aerosols, in the absence of nicotine and flavors, impact parameters of mucociliary function in human volunteers, a large animal model (sheep), and air-liquid interface (ALI) cultures of primary human bronchial epithelial cells (HBECs). We found that VG-containing (VG or PG/VG), but not sole PG-containing, e-cig aerosols reduced the activity of nasal cystic fibrosis transmembrane conductance regulator (CFTR) in human volunteers who vaped for seven days. Markers of inflammation, including interleukin-6 (IL6), interleukin-8 (IL8) and matrix metalloproteinase-9 (MMP9) mRNAs, as well as MMP-9 activity and mucin 5AC (MUC5AC) expression levels, were also elevated in nasal samples from volunteers who vaped VG-containing e-liquids. In sheep, exposures to VG e-cig aerosols for five days increased mucus concentrations and MMP-9 activity in tracheal secretions and plasma levels of transforming growth factor-beta 1 (TGF-β1). In vitro exposure of HBECs to VG e-cig aerosols for five days decreased ciliary beating and increased mucus concentrations. VG e-cig aerosols also reduced CFTR function in HBECs, mechanistically by reducing membrane fluidity. Although VG e-cig aerosols did not increase MMP9 mRNA expression, expression levels of IL6, IL8, TGFB1, and MUC5AC mRNAs were significantly increased in HBECs after seven days of exposure. Thus, VG e-cig aerosols can potentially cause harm in the airway by inducing inflammation and ion channel dysfunction with consequent mucus hyperconcentration.
Keywords: Airway epithelium; CFTR; Mucus; e-cigarette; vegetable glycerin.
Copyright © 2022 Kim, Chung, Dennis, Yoshida, Aguiar, Aller, Mendes, Schmid, Sabater, Baumlin and Salathe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anderson W. H., Coakley R. D., Button B., Henderson A. G., Zeman K. L., Alexis N. E., et al. (2015). The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 192 (2), 182–190. 10.1164/rccm.201412-2230OC - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

