Prediction of tumor recurrence by distinct immunoprofiles in liver transplant patients based on mass cytometry
- PMID: 36225628
- PMCID: PMC9548010
Prediction of tumor recurrence by distinct immunoprofiles in liver transplant patients based on mass cytometry
Abstract
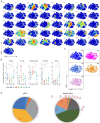
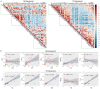
Recurrence of hepatocellular carcinoma (HCC) after liver transplantation (LT) is a marker of poor prognosis. However, the reliable biomarkers of post-LT HCC recurrence remain to be identified. In this study, serial peripheral blood samples from the LT recipients with and without HCC recurrence were collected at five time points. Single-cell mass cytomertry (CyTOF) was utilized for the in-depth analysis of peripheral blood monocellular cells (PBMCs). CyTOF analysis showed that at 3 weeks post-LT, the activated immune cell population was increased, while the fraction of immune cells with suppressive functions (myeloid-derived suppressive cells) was reduced. The post-LT immune composition in patients with LT for HCC was enormously different from that in patients with LT for causes other than HCC. Furthermore, at 3 weeks after LT, compared with patients without recurrence, the patients with HCC recurrences were high in two subsets of T cells: CD57+ HLA-DR+ CD8+ and CD28+γδ. The CD57+ HLA-DR+ CD8+ T cells presented high levels of perforin, granzyme B, and Ki-67 and displayed a highly cytotoxic and proliferative phenotype, while the CD28+γδ T cells had reduced levels of IFN-γ and, hence, were less activated compared to CD28- cells. Based on these findings, we concluded that analyzing the PBMCs of LT recipients by CyTOF can predict the post-LT HCC recurrence. The distinct immune features can stratify patients with the risk of HCC recurrence at 3 weeks after LT, which will help clinician in further management plan and improve the prognosis of patients.
Keywords: Hepatocellular carcinoma; liver transplantation; mass cytometry.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures





References
-
- Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–217. - PubMed
-
- Yang Z, Wang S, Tian XY, Xie QF, Zhuang L, Li QY, Chen CZ, Zheng SS. Impact of treatment modalities on patients with recurrent hepatocellular carcinoma after liver transplantation: preliminary experience. Hepatobiliary Pancreat Dis Int. 2020;19:365–370. - PubMed
-
- Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
