Investigation of the effects of downregulation of jumping translocation breakpoint (JTB) protein expression in MCF7 cells for potential use as a biomarker in breast cancer
- PMID: 36225631
- PMCID: PMC9548009
Investigation of the effects of downregulation of jumping translocation breakpoint (JTB) protein expression in MCF7 cells for potential use as a biomarker in breast cancer
Abstract
MCF7 is a commonly used luminal type A non-invasive/poor-invasive human breast cancer cell line that does not usually migrate or invade compared with MDA-MB-231 highly metastatic cells, which emphasize an invasive and migratory behavior. Under special conditions, MCF7 cells might acquire invasive features. The aberration in expression and biological functions of the jumping translocation breackpoint (JTB) protein is associated with malignant transformation of cells, based on mitochondrial dysfunction, inhibition of tumor suppressive function of TGF-β, and involvement in cancer cell cycle. To investigate new putative functions of JTB by cellular proteomics, we analyzed the biological processes and pathways that are associated with the JTB protein downregulation. The results demonstrated that MCF7 cell line developed a more "aggressive" phenotype and behavior. Most of the proteins that were overexpressed in this experiment promoted the actin cytoskeleton reorganization that is involved in growth and metastatic dissemination of cancer cells. Some of these proteins are involved in the epithelial-mesenchymal transition (EMT) process (ACTBL2, TUBA4A, MYH14, CSPG5, PKM, UGDH, HSP90AA2, and MIF), in correlation with the energy metabolism reprogramming (PKM, UGDH), stress-response (HSP10, HSP70A1A, HSP90AA2), and immune and inflammatory response (MIF and ERp57-TAPBP). Almost all upregulated proteins in JTB downregulated condition promote viability, motility, proliferation, invasion, survival into a hostile microenvironment, metabolic reprogramming, and escaping of tumor cells from host immune control, leading to a more invasive phenotype for MCF7 cell line. Due to their downregulated condition, four proteins, such as CREBZF, KMT2B, SELENOS and CACNA1I are also involved in maintenance of the invasive phenotype of cancer cells, promoting cell proliferation, migration, invasion and tumorigenesis. Other downregulated proteins, such as MAZ, PLEKHG2, ENO1, TPI2, TOR2A, and CNNM1, may promote suppression of cancer cell growth, invasion, EMT, tumorigenic abilities, interacting with glucose and lipid metabolism, disrupting nuclear envelope stability, or suppressing apoptosis and developing anti-angiogenetic activities. Therefore, the main biological processes and pathways that may increase the tumorigenic potential of the MCF7 cells in JTB downregulated condition are related to the actin cytoskeleton organization, EMT, mitotic cell cycle, glycolysis and fatty acid metabolism, inflammatory response and macrophage activation, chemotaxis and migration, cellular response to stress condition (oxidative stress and hypoxia), transcription control, histone modification and ion transport.
Keywords: Breast cancer; JTB downregulated condition; jumping translocation breakpoint (JTB) protein; proteomics.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures
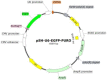

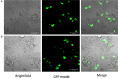

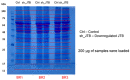
Similar articles
-
Investigating the Function of Human Jumping Translocation Breakpoint Protein (hJTB) and Its Interacting Partners through In-Solution Proteomics of MCF7 Cells.Molecules. 2022 Nov 28;27(23):8301. doi: 10.3390/molecules27238301. Molecules. 2022. PMID: 36500393 Free PMC article.
-
Investigation of the effects of overexpression of jumping translocation breakpoint (JTB) protein in MCF7 cells for potential use as a biomarker in breast cancer.Am J Cancer Res. 2022 Apr 15;12(4):1784-1823. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530281 Free PMC article.
-
Two-Dimensional-PAGE Coupled with nLC-MS/MS-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in MCF7 Breast Cancer Cells Transfected for JTB Protein Silencing.Molecules. 2023 Nov 9;28(22):7501. doi: 10.3390/molecules28227501. Molecules. 2023. PMID: 38005222 Free PMC article.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
-
Characterization of LIMA1 and its emerging roles and potential therapeutic prospects in cancers.Front Oncol. 2023 May 19;13:1115943. doi: 10.3389/fonc.2023.1115943. eCollection 2023. Front Oncol. 2023. PMID: 37274282 Free PMC article. Review.
Cited by
-
Proteomics-Based Identification of Dysregulated Proteins in Breast Cancer.Proteomes. 2022 Oct 21;10(4):35. doi: 10.3390/proteomes10040035. Proteomes. 2022. PMID: 36278695 Free PMC article. Review.
-
Proteomics Analysis of Lymphoblastoid Cell Lines from Patients with Amyotrophic Lateral Sclerosis.Molecules. 2023 Feb 21;28(5):2014. doi: 10.3390/molecules28052014. Molecules. 2023. PMID: 36903260 Free PMC article.
-
From Jumping Gene to Cancer: Revisiting the Role of JTB Protein.Biomedicines. 2025 Jul 12;13(7):1705. doi: 10.3390/biomedicines13071705. Biomedicines. 2025. PMID: 40722776 Free PMC article. Review.
-
Omics-Based Investigations of Breast Cancer.Molecules. 2023 Jun 14;28(12):4768. doi: 10.3390/molecules28124768. Molecules. 2023. PMID: 37375323 Free PMC article. Review.
-
PLEKHG2 Promotes NSCLC Cell Growth by Increasing Glycolysis via Activated PI3K/AKT Pathway.J Cancer. 2023 Oct 30;14(18):3550-3560. doi: 10.7150/jca.88857. eCollection 2023. J Cancer. 2023. PMID: 38021149 Free PMC article.
References
-
- Kanome T, Itoh N, Ishikawa F, Mori K, Kim-Kaneyama JR, Nose K, Shibanuma M. Characterization of jumping translocation breakpoint (JTB) gene product isolated as a TGF-β1-inducible clone involved in regulation of mitochondrial function, cell growth and cell death. Oncogene. 2007;26:5991–6001. - PubMed
-
- Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. - PubMed
-
- Platica M, Ionescu A, Ivan E, Holland JF, Mandeli J, Platica O. PAR, a protein involved in the cell cycle, is functionally related to chromosomal passenger proteins. Int J Oncol. 2011;38:777–785. - PubMed
-
- Rousseau F, Pan B, Fairbrother WJ, Bazan JF, Lingel A. The structure of the extracellular domain of the jumping translocation breakpoint protein reveals a variation of the midkine fold. J Mol Biol. 2012;415:22–28. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
