Ability of donkey sperm to tolerate cooling: Effect of extender base and removal of seminal plasma on sperm parameters and fertility rates in mares
- PMID: 36225802
- PMCID: PMC9548546
- DOI: 10.3389/fvets.2022.1011899
Ability of donkey sperm to tolerate cooling: Effect of extender base and removal of seminal plasma on sperm parameters and fertility rates in mares
Abstract
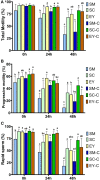
Artificial insemination using cooled-transported semen has marked importance in equine breeding programs around the world, and the high value of mules has generated avid interest in donkey semen biotechnology. However, donkey semen cools poorly in commercially available equine extenders. Therefore, this study aimed to develop approaches to improve the ability of donkey semen to tolerate cooling. Ejaculates of seven donkeys (n = 21) were cooled at 5°C for 48 h in three different extenders (milk-based, SM; sodium caseinate-based, SC; or egg yolk-based, EY) in the presence or absence of seminal plasma (centrifugation, C). Sperm motility, plasma membrane integrity (PMI), plasma membrane stability (PMS), mitochondrial membrane potential (HMMP), intracellular hydrogen peroxide (H2O2), and intracellular superoxide ( ) were assessed before, 24 h, and 48 h post-cooling. In addition, 15 mares (163 estrous cycles) were randomly inseminated with semen from two jacks (Jack 1, n = 90; Jack 2, n = 73) previously cooled for 24 h under one of the treatments (SM, SC, EY, SM-C, SC-C, or EY-C). Groups EY, SC-C, and EY-C (P < 0.05) demonstrated superior sperm analytical parameters to SM at 24 and 48 h. Centrifugation positively affected sperm analytical parameters in cooled donkey semen extended in SM and SC (P < 0.05). Mares bred with semen extended in SC (67%, 18/27), SC-C (89%, 24/27), EY (89%, 25/28), or EY-C (74%, 20/27) had significantly greater conception rates than mares bred with SM (33%, 9/27; P < 0.05). Mares bred with SM-C had intermediate conception rates (59%, 16/27). In conclusion, SC and EY improved the cooling ability and fertility of donkey semen in horse mares, and centrifugation positively affected donkey semen extended in SM.
Keywords: artificial insemination; equine; jack; mule; stallion.
Copyright © 2022 Gobato, Segabinazzi, Scheeren, Bandeira, Freitas-Dell'Aqua, Dell'Aqua and Papa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Post-cooling semen processing and sperm re-suspension as an alternative method to circumvent poor semen cooling in stallions.Equine Vet J. 2024 Jul;56(4):697-710. doi: 10.1111/evj.14085. Epub 2024 Apr 3. Equine Vet J. 2024. PMID: 38567428
-
Post-cooling sperm processing can rescue sperm quality of cooled-stored stallion semen.Theriogenology. 2024 Oct 1;227:21-30. doi: 10.1016/j.theriogenology.2024.07.004. Epub 2024 Jul 8. Theriogenology. 2024. PMID: 38996526
-
Cholesterol-Loaded Cyclodextrin Addition to Skim Milk-Based Extender Enhances Donkey Semen Cooling and Fertility in Horse Mares.J Equine Vet Sci. 2021 Oct;105:103719. doi: 10.1016/j.jevs.2021.103719. Epub 2021 Jul 18. J Equine Vet Sci. 2021. PMID: 34607680
-
Low dose insemination of mares using non-sorted and sex-sorted sperm.Anim Reprod Sci. 2001 Dec 3;68(3-4):279-89. doi: 10.1016/s0378-4320(01)00165-8. Anim Reprod Sci. 2001. PMID: 11744272 Review.
-
The equine frozen semen industry.Anim Reprod Sci. 2001 Dec 3;68(3-4):191-200. doi: 10.1016/s0378-4320(01)00156-7. Anim Reprod Sci. 2001. PMID: 11744264 Review.
Cited by
-
Current and Emerging Advanced Techniques for Breeding Donkeys and Mules.Animals (Basel). 2025 Mar 29;15(7):990. doi: 10.3390/ani15070990. Animals (Basel). 2025. PMID: 40218383 Free PMC article. Review.
-
Comprehensive Integrated Analyses of Proteins and Metabolites in Equine Seminal Plasma (Horses and Donkeys).Proteomes. 2025 Jul 4;13(3):33. doi: 10.3390/proteomes13030033. Proteomes. 2025. PMID: 40700277 Free PMC article.
References
-
- Camillo F, Rota A, Biagini L, Tesi M, Fanelli D, Panzani D. The current situation and trend of donkey industry in Europe. J Equine Vet Sci. (2018) 65:44–9. 10.1016/j.jevs.2017.11.008 - DOI
-
- Santos GF, Henry M, Sampaio IBM, Gastal EL. Effect of Cooling system and rate of cooling on sperm quality of donkey semen preserved at 5°C. Biol Reprod. (1995) 52:761–7. 10.1093/biolreprod/52.monograph_series1.761 - DOI
-
- Cottorello ACP, Amancio RC, Henry M, Borges I. Effect of storage temperature and extenders on “in vitro” activity of donkey spermatozoa. Theriogenology. (2002) 58:325–8. 10.1016/S0093-691X(02)00753-7 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

