Comparative analysis of the neutralizing activity against SARS-CoV-2 Wuhan-Hu-1 strain and variants of concern: Performance evaluation of a pseudovirus-based neutralization assay
- PMID: 36225911
- PMCID: PMC9549111
- DOI: 10.3389/fimmu.2022.981693
Comparative analysis of the neutralizing activity against SARS-CoV-2 Wuhan-Hu-1 strain and variants of concern: Performance evaluation of a pseudovirus-based neutralization assay
Abstract
Objectives: Emergence of new variants of SARS-CoV-2 might affect vaccine efficacy. Therefore, assessing the capacity of sera to neutralize variants of concern (VOCs) in BSL-2 conditions will help evaluating the immune status of population following vaccination or infection.
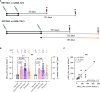
Methods: Pseudotyped viruses bearing SARS-CoV-2 spike protein from Wuhan-Hu-1/D614G strains (wild type, WT), B.1.617.2 (Delta), or B.1.1.529 (Omicron) VOCs were generated to assess the neutralizing antibodies (nAbs) activity by a pseudovirus-based neutralization assay (PVNA). PVNA performance was assessed in comparison to the micro-neutralization test (MNT) based on live viruses. Sera collected from COVID-19 convalescents and vaccinees receiving mRNA (BNT16b2 or mRNA-1273) or viral vector (AZD1222 or Ad26.COV2.S) vaccines were used to measure nAbs elicited by two-dose BNT16b2, mRNA-1273, AZD1222 or one-dose Ad26.CO2.S, at different times from completed vaccination, ~ 1.5 month and ~ 4-6 months. Sera from pre-pandemic and unvaccinated individuals were analyzed as controls. Neutralizing activity following booster vaccinations against VOCs was also determined.
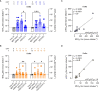
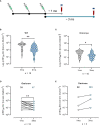
Results: PVNA titers correlated with the gold standard MNT assay, validating the reliability of PVNA. Sera analyzed late from the second dose showed a reduced neutralization activity compared to sera collected earlier. Ad26.CO2.S vaccination led to very low or absent nAbs. Neutralization of Delta and Omicron BA.1 VOCs showed significant reduction of nAbs respect to WT strain. Importantly, booster doses enhanced Omicron BA.1 nAbs, with persistent levels at 3 months from boosting.
Conclusions: PVNA is a reliable tool for assessing anti-SARS-CoV-2 nAbs helping the establishment of a correlate of protection and the management of vaccination strategies.
Keywords: COVID-19; SARS-CoV-2; immunogenicity; neutralization assay; neutralizing antibodies; pseudotyped virus; vaccines; variants of concern.
Copyright © 2022 D’Apice, Trovato, Gramigna, Colavita, Francalancia, Matusali, Meschi, Lapa, Bettini, Mizzoni, Aurisicchio, Di Caro, Castilletti and De Berardinis.
Conflict of interest statement
Author LA was employed by company Takis Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

