Study of necrotic apoptosis by pulsed electric field ablation in rabbit left ventricular myocardium
- PMID: 36225956
- PMCID: PMC9548611
- DOI: 10.3389/fcvm.2022.1012020
Study of necrotic apoptosis by pulsed electric field ablation in rabbit left ventricular myocardium
Abstract
Objective: We investigate the characteristics of histological damage to myocardial cells in the ablation region and surrounding areas of the left ventricular epicardium in rabbits using our self-developed cardiac pulsed electric field (PEF) ablation instrument and ablation catheter.
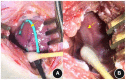
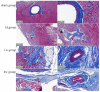
Methods: Forty eight New Zealand rabbits underwent ablation on the left ventricular myocardium after open-heart exposure with a cardiac arrhythmia PEF ablation device and ablation catheter developed by the Medical Translation Laboratory of Pulsed Electric Field Technology in Zhejiang Province. The ablation parameters were set as biphasic electrical pulses; voltage, ±800 V; pulse width, 10 μs; interphase delay, 500 us. Six rabbits were included in the sham group and 42 other rabbits were randomly divided into immediately, 6-h, 1-, 3-day, 1-, 2-, and 4-week post-ablation groups, with six rabbits in each group. Creatine kinase- (CK)-MB isoenzyme (CK-MB), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels were measured before and at different time points after PEF ablation to analyze their dynamic evolution. Masson staining of tissue block sections of left ventricular myocardial ablation and adjacent tissue heart specimens was performed, and the occurrence of TUNEL apoptosis in myocardium tissue was analyzed.
Results: All rabbits completed the PEF ablation procedure and the follow-up process. After PEF ablation, the levels of cardiac enzymes, including CK-MB, CK, and AST, increased significantly, peaking 1-3 days after the procedure. In particular, those of CK and CK-MB increased by 15-20 times but returned to the preoperative level after 2 weeks. Based on general observation, it was found that the myocardium in the ablation area was swollen immediately after PEF ablation. Masson staining analysis revealed that cardiomyocytes were broken and infiltrated by erythrocytes after 6 h. After 1 day, the cells started to experience atrophy and necrosis; after 3 days, fibrotic replacement of the necrotic area became obvious. Then, by 4 weeks, the myocardial cells were completely replaced by hyperplasia. Apoptosis occurred significantly at 6 h and peaked at 24 h post-ablation, demonstrating a 37.7-fold increase; apoptotic cell counts decreased significantly at 3 days post-ablation, and no significant apoptotic cardiomyocytes were seen after 1 week.
Conclusion: After PEF ablation, cardiomyocytes showed apoptotic process and dyed, at least partially, through a secondary necrosis, the ablation boundary was clear, the ablation area was replaced by structurally intact fibroblasts, no island myocardium tissue were seen, and the ablation area vessels and nerves were not affected.
Keywords: ablation; cardiovascular pathology; heart; necrotizing apoptosis; pulsed electric field; rabbit.
Copyright © 2022 Zhao, Chen, Wu, Qiu, Hong, Chen and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Tang Min NG, Jinrui G, Yu Q, Yulong G, Ke L, Fengyuan Y, et al. . Novel pulsed field ablation system in treatment of paroxysmal atrial fibrillation: 3 cases. Chin J Cardiac Arrhyth. (2022) 26:96–8. 10.3760/cma.j.cn113859-20211203-00243 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

