Fully automatic cardiac four chamber and great vessel segmentation on CT pulmonary angiography using deep learning
- PMID: 36225963
- PMCID: PMC9549370
- DOI: 10.3389/fcvm.2022.983859
Fully automatic cardiac four chamber and great vessel segmentation on CT pulmonary angiography using deep learning
Abstract
Introduction: Computed tomography pulmonary angiography (CTPA) is an essential test in the work-up of suspected pulmonary vascular disease including pulmonary hypertension and pulmonary embolism. Cardiac and great vessel assessments on CTPA are based on visual assessment and manual measurements which are known to have poor reproducibility. The primary aim of this study was to develop an automated whole heart segmentation (four chamber and great vessels) model for CTPA.
Methods: A nine structure semantic segmentation model of the heart and great vessels was developed using 200 patients (80/20/100 training/validation/internal testing) with testing in 20 external patients. Ground truth segmentations were performed by consultant cardiothoracic radiologists. Failure analysis was conducted in 1,333 patients with mixed pulmonary vascular disease. Segmentation was achieved using deep learning via a convolutional neural network. Volumetric imaging biomarkers were correlated with invasive haemodynamics in the test cohort.
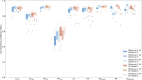
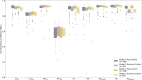
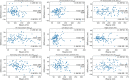
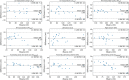
Results: Dice similarity coefficients (DSC) for segmented structures were in the range 0.58-0.93 for both the internal and external test cohorts. The left and right ventricle myocardium segmentations had lower DSC of 0.83 and 0.58 respectively while all other structures had DSC >0.89 in the internal test cohort and >0.87 in the external test cohort. Interobserver comparison found that the left and right ventricle myocardium segmentations showed the most variation between observers: mean DSC (range) of 0.795 (0.785-0.801) and 0.520 (0.482-0.542) respectively. Right ventricle myocardial volume had strong correlation with mean pulmonary artery pressure (Spearman's correlation coefficient = 0.7). The volume of segmented cardiac structures by deep learning had higher or equivalent correlation with invasive haemodynamics than by manual segmentations. The model demonstrated good generalisability to different vendors and hospitals with similar performance in the external test cohort. The failure rates in mixed pulmonary vascular disease were low (<3.9%) indicating good generalisability of the model to different diseases.
Conclusion: Fully automated segmentation of the four cardiac chambers and great vessels has been achieved in CTPA with high accuracy and low rates of failure. DL volumetric biomarkers can potentially improve CTPA cardiac assessment and invasive haemodynamic prediction.
Keywords: computed tomography pulmonary angiography (CTPA); deep-learning (DL); pulmonary vascular disease (PVD); semantic segmentation and labelling; whole heart segmentation.
Copyright © 2022 Sharkey, Taylor, Alabed, Dwivedi, Karunasaagarar, Johns, Rajaram, Garg, Alkhanfar, Metherall, O'Regan, van der Geest, Condliffe, Kiely, Mamalakis and Swift.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Deep learning from dual-energy information for whole-heart segmentation in dual-energy and single-energy non-contrast-enhanced cardiac CT.Med Phys. 2020 Oct;47(10):5048-5060. doi: 10.1002/mp.14451. Epub 2020 Aug 27. Med Phys. 2020. PMID: 32786071
-
Utility of Automated Cardiac Chamber Volumetry by Nongated CT Pulmonary Angiography for Detection of Pulmonary Hypertension Using the 2018 Updated Hemodynamic Definition.AJR Am J Roentgenol. 2022 Jul;219(1):66-75. doi: 10.2214/AJR.21.27147. Epub 2022 Jan 26. AJR Am J Roentgenol. 2022. PMID: 35080457
-
Two-stage deep learning model for fully automated pancreas segmentation on computed tomography: Comparison with intra-reader and inter-reader reliability at full and reduced radiation dose on an external dataset.Med Phys. 2021 May;48(5):2468-2481. doi: 10.1002/mp.14782. Epub 2021 Mar 16. Med Phys. 2021. PMID: 33595105
-
Advancements in cardiac structures segmentation: a comprehensive systematic review of deep learning in CT imaging.Front Cardiovasc Med. 2024 Jan 22;11:1323461. doi: 10.3389/fcvm.2024.1323461. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38317865 Free PMC article. Review.
-
Automated vessel segmentation in lung CT and CTA images via deep neural networks.J Xray Sci Technol. 2021;29(6):1123-1137. doi: 10.3233/XST-210955. J Xray Sci Technol. 2021. PMID: 34421004 Review.
Cited by
-
Systematic pulmonary embolism follow-up increases diagnostic rates of chronic thromboembolic pulmonary hypertension and identifies less severe disease: results from the ASPIRE Registry.Eur Respir J. 2024 Mar 14;63(3):2300846. doi: 10.1183/13993003.00846-2023. Print 2024 Mar. Eur Respir J. 2024. PMID: 38302154 Free PMC article.
-
Comprehensive review of pulmonary embolism imaging: past, present and future innovations in computed tomography (CT) and other diagnostic techniques.Jpn J Radiol. 2025 Jun 28. doi: 10.1007/s11604-025-01811-8. Online ahead of print. Jpn J Radiol. 2025. PMID: 40580273 Review.
-
Cardiac Fibrosis Automated Diagnosis Based on FibrosisNet Network Using CMR Ischemic Cardiomyopathy.Diagnostics (Basel). 2024 Jan 24;14(3):255. doi: 10.3390/diagnostics14030255. Diagnostics (Basel). 2024. PMID: 38337771 Free PMC article.
-
Emerging multimodality imaging techniques for the pulmonary circulation.Eur Respir J. 2024 Oct 31;64(4):2401128. doi: 10.1183/13993003.01128-2024. Print 2024 Oct. Eur Respir J. 2024. PMID: 39209480 Free PMC article. Review.
-
Fully Automated Assessment of Cardiac Chamber Volumes and Myocardial Mass on Non-Contrast Chest CT with a Deep Learning Model: Validation Against Cardiac MR.Diagnostics (Basel). 2024 Dec 21;14(24):2884. doi: 10.3390/diagnostics14242884. Diagnostics (Basel). 2024. PMID: 39767245 Free PMC article.
References
-
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. . 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Resp J. (2019) 54:1901647. 10.1183/13993003.01647-2019 - DOI - PubMed
-
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J. (2015) 46:903–75, 1855–6. 10.1183/13993003.51032-2015 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

