Pharmacogenetic inhibition of TrkB signaling in adult mice attenuates mechanical hypersensitivity and improves locomotor function after spinal cord injury
- PMID: 36226073
- PMCID: PMC9548551
- DOI: 10.3389/fncel.2022.987236
Pharmacogenetic inhibition of TrkB signaling in adult mice attenuates mechanical hypersensitivity and improves locomotor function after spinal cord injury
Abstract
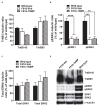
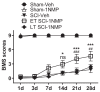
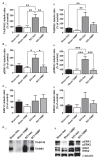
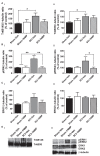
Brain-derived neurotrophic factor (BDNF) signals through tropomyosin receptor kinase B (TrkB), to exert various types of plasticity. The exact involvement of BDNF and TrkB in neuropathic pain states after spinal cord injury (SCI) remains unresolved. This study utilized transgenic TrkBF616 mice to examine the effect of pharmacogenetic inhibition of TrkB signaling, induced by treatment with 1NM-PP1 (1NMP) in drinking water for 5 days, on formalin-induced inflammatory pain, pain hypersensitivity, and locomotor dysfunction after thoracic spinal contusion. We also examined TrkB, ERK1/2, and pERK1/2 expression in the lumbar spinal cord and trunk skin. The results showed that formalin-induced pain responses were robustly attenuated in 1NMP-treated mice. Weekly assessment of tactile sensitivity with the von Frey test showed that treatment with 1NMP immediately after SCI blocked the development of mechanical hypersensitivity up to 4 weeks post-SCI. Contrastingly, when treatment started 2 weeks after SCI, 1NMP reversibly and partially attenuated hind-paw hypersensitivity. Locomotor scores were significantly improved in the early-treated 1NMP mice compared to late-treated or vehicle-treated SCI mice. 1NMP treatment attenuated SCI-induced increases in TrkB and pERK1/2 levels in the lumbar cord but failed to exert similar effects in the trunk skin. These results suggest that early onset TrkB signaling after SCI contributes to maladaptive plasticity that leads to spinal pain hypersensitivity and impaired locomotor function.
Keywords: BDNF; TrkB; mechanical hypersensitivity; pERK; plasticity; spinal cord injury.
Copyright © 2022 Martin, Noble, Parvin, Jang and Garraway.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
TrkB Agonist (7,8-DHF)-Induced Responses in Dorsal Root Ganglia Neurons Are Decreased after Spinal Cord Injury: Implication for Peripheral Pain Mechanisms.eNeuro. 2025 Jan 3;12(1):ENEURO.0219-24.2024. doi: 10.1523/ENEURO.0219-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39753357 Free PMC article.
-
Truncated TrkB.T1-Mediated Astrocyte Dysfunction Contributes to Impaired Motor Function and Neuropathic Pain after Spinal Cord Injury.J Neurosci. 2017 Apr 5;37(14):3956-3971. doi: 10.1523/JNEUROSCI.3353-16.2017. Epub 2017 Mar 7. J Neurosci. 2017. PMID: 28270575 Free PMC article.
-
TrkB.T1 contributes to neuropathic pain after spinal cord injury through regulation of cell cycle pathways.J Neurosci. 2013 Jul 24;33(30):12447-63. doi: 10.1523/JNEUROSCI.0846-13.2013. J Neurosci. 2013. PMID: 23884949 Free PMC article.
-
Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury.Mol Pain. 2020 Jan-Dec;16:1744806920924511. doi: 10.1177/1744806920924511. Mol Pain. 2020. PMID: 32418502 Free PMC article.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
Cited by
-
Sodium Bicarbonate Decreases Alcohol Consumption in Mice.Int J Mol Sci. 2024 May 3;25(9):5006. doi: 10.3390/ijms25095006. Int J Mol Sci. 2024. PMID: 38732226 Free PMC article.
-
TrkB Agonist (7,8-DHF)-Induced Responses in Dorsal Root Ganglia Neurons Are Decreased after Spinal Cord Injury: Implication for Peripheral Pain Mechanisms.eNeuro. 2025 Jan 3;12(1):ENEURO.0219-24.2024. doi: 10.1523/ENEURO.0219-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39753357 Free PMC article.
-
A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain.Neurobiol Pain. 2024 Jan 20;15:100151. doi: 10.1016/j.ynpai.2024.100151. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38314104 Free PMC article. Review.
-
Diet-microbiome interactions promote enteric nervous system resilience following spinal cord injury.NPJ Biofilms Microbiomes. 2024 Aug 29;10(1):75. doi: 10.1038/s41522-024-00556-y. NPJ Biofilms Microbiomes. 2024. PMID: 39209925 Free PMC article.
-
Brain-Derived Neurotrophic Factor, Nociception, and Pain.Biomolecules. 2024 Apr 30;14(5):539. doi: 10.3390/biom14050539. Biomolecules. 2024. PMID: 38785946 Free PMC article. Review.
References
-
- Akhter E. T., Griffith R. W., English A. W., Alvarez F. J. (2019). Removal of the potassium chloride co-transporter from the somatodendritic membrane of axotomized motoneurons is independent of BDNF/TrkB signaling but is controlled by neuromuscular innervation. eNeuro 6:ENEURO.0172-19.2019. 10.1523/ENEURO.0172-19.2019 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

