Combined brain-derived neurotrophic factor and neurotrophin-3 treatment is preferred over either one separately in the preservation of the auditory nerve in deafened guinea pigs
- PMID: 36226314
- PMCID: PMC9549372
- DOI: 10.3389/fnmol.2022.935111
Combined brain-derived neurotrophic factor and neurotrophin-3 treatment is preferred over either one separately in the preservation of the auditory nerve in deafened guinea pigs
Abstract
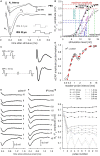
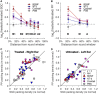
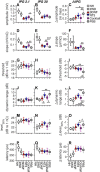
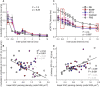
Severe hearing loss or deafness is often caused by cochlear hair cell loss and can be mitigated by a cochlear implant (CI). CIs target the auditory nerve, consisting of spiral ganglion cells (SGCs), which degenerate gradually, following hair cell loss. In animal models, it has been established that treatment with the neurotrophins brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) reduce SGC degeneration. In this study, we aimed to investigate whether treatment with both BDNF and NT-3 (Cocktail) is superior to treatment with each neurotrophin separately regarding cell preservation and neural responsiveness to electrical stimulation. To this end, deafened guinea pigs received neurotrophic treatment in their right ear via a gelatin sponge on the perforated round window membrane, followed by cochlear implantation 4 weeks later in the same ear for electrophysiological recordings to various stimulation paradigms. Normal-hearing and deafened untreated guinea pigs were included as positive and negative controls, respectively. Substantial SGC loss occurred in all deafened animals. Each of the neurotrophic treatments led to enhanced SGC survival mainly in the basal turn of the cochlea, gradually decreasing toward the apex. The Cocktail treatment resulted in the highest SGC survival in the treated ear, followed by BDNF, with the least protection of SGCs following NT-3 treatment. Survival of the SGC's peripheral processes (PPs) followed the same trend in response to the treatment. However, survival of SGCs and PPs in the contralateral untreated ears was also highest in the Cocktail group. Consequently, analysis of the ratio between the treated and untreated ears showed that the BDNF group, which showed low SGC survival in the untreated ear, had the highest relative SGC survival of the three neurotrophin-treated groups. Neurotrophic treatment had positive effects in part of the electrically evoked compound action-potential recording paradigms. These effects were only observed for the BDNF or Cocktail treatment. We conclude that treatment with either BDNF or a cocktail of BDNF and NT-3 is preferred to NT-3 alone. Furthermore, since the Cocktail treatment resulted in better electrophysiological responsiveness and overall higher SGC survival than BDNF alone, we are inclined to recommend the Cocktail treatment rather than BDNF alone.
Keywords: cochlea; cochlear implant; eCAP; hearing loss; neurodegeneration; neuroprotection; neurotrophin.
Copyright © 2022 Vink, Ramekers, Thomeer and Versnel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The efficacy of a TrkB monoclonal antibody agonist in preserving the auditory nerve in deafened guinea pigs.Hear Res. 2023 Nov;439:108895. doi: 10.1016/j.heares.2023.108895. Epub 2023 Oct 4. Hear Res. 2023. PMID: 37837701
-
Temporary Neurotrophin Treatment Prevents Deafness-Induced Auditory Nerve Degeneration and Preserves Function.J Neurosci. 2015 Sep 9;35(36):12331-45. doi: 10.1523/JNEUROSCI.0096-15.2015. J Neurosci. 2015. PMID: 26354903 Free PMC article.
-
BDNF Outperforms TrkB Agonist 7,8,3'-THF in Preserving the Auditory Nerve in Deafened Guinea Pigs.Brain Sci. 2020 Oct 28;10(11):787. doi: 10.3390/brainsci10110787. Brain Sci. 2020. PMID: 33126525 Free PMC article.
-
Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness.Hear Res. 2020 Sep 1;394:107955. doi: 10.1016/j.heares.2020.107955. Epub 2020 Apr 5. Hear Res. 2020. PMID: 32331858 Free PMC article. Review.
-
Neurotrophins and their role in the cochlea.Hear Res. 2012 Jun;288(1-2):19-33. doi: 10.1016/j.heares.2012.03.002. Epub 2012 Mar 21. Hear Res. 2012. PMID: 22465680 Review.
Cited by
-
Optimizing Factors in Murine Whole-Organ Cochlea Culture.Int J Mol Sci. 2025 Apr 21;26(8):3908. doi: 10.3390/ijms26083908. Int J Mol Sci. 2025. PMID: 40332783 Free PMC article.
-
Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial?Int J Mol Sci. 2023 Jan 19;24(3):2013. doi: 10.3390/ijms24032013. Int J Mol Sci. 2023. PMID: 36768339 Free PMC article.
-
Regeneration of Hair Cells from Endogenous Otic Progenitors in the Adult Mammalian Cochlea: Understanding Its Origins and Future Directions.Int J Mol Sci. 2023 Apr 25;24(9):7840. doi: 10.3390/ijms24097840. Int J Mol Sci. 2023. PMID: 37175547 Free PMC article. Review.
References
-
- Agterberg M. J. H., Versnel H., de Groot J. C. M. J., Smoorenburg G. F., Albers F. W. J., Klis S. F. L. (2008). Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear. Res. 244 25–34. 10.1016/j.heares.2008.07.004 - DOI - PubMed
-
- Agterberg M. J. H., Versnel H., van Dijk L. M., de Groot J. C. M. J., Klis S. F. L. (2009). Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J. Assoc. Res. Otolaryngol. 10 355–367. 10.1007/s10162-009-0170-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

