Efficacy and safety of sotrovimab in patients with COVID-19: A rapid review and meta-analysis
- PMID: 36226323
- PMCID: PMC9874927
- DOI: 10.1002/rmv.2402
Efficacy and safety of sotrovimab in patients with COVID-19: A rapid review and meta-analysis
Abstract
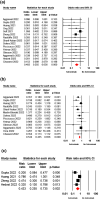
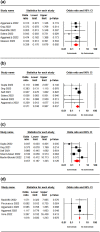
The therapeutic potential of sotrovimab in the treatment of coronavirus disease 2019 (COVID-19) is a controversial issue. The aim of this study was to evaluate the efficacy and safety of sotrovimab in COVID-19 patients. To this end, PubMed, Cochrane Library, Embase, Web of Science, medRxiv, and Google Scholar were searched up to 15 August 2022. The reference lists of key studies were also scanned to find additional records. Meta-analysis was performed using Comprehensive Meta-Analysis. Seventeen studies involving 27,429 patients were included. A significant difference was observed in mortality rate (odds ratio [OR] = 0.40; 95% CI: 0.25-0.63, p = 0.00), hospitalisation rate (OR = 0.53; 95% CI: 0.43-0.65. p = 0.00), hospital or death rate (OR = 0.43; 95% CI: 0.25-0.73, p = 0.00), the need for mechanical ventilation (OR = 0.57; 95% CI: 0.33-0.96, p = 0.03), and ICU admission (OR = 0.33; 95% CI: 0.17-0.67, p = 0.00) of the sotrovimab-receiving group compared to those having no sotrovimab. However, no significant difference was observed between the two groups in terms of disease progression (OR = 0.45; 95% CI: 0.16-1.24, p = 0.12) and emergency department visit (OR = 1.01; 95% CI: 0.83-1.24, p = 0.87). The two groups had no significant difference in terms of incidence of adverse events (OR = 0.98; 95% CI: 0.78-1.23, p = 0.88). The findings of the present meta-analysis support that sotrovimab could be an effective and safe treatment option to reduce mortality and hospitalisation rate in both Delta and Omicron Variants of COVID-19.
Keywords: COVID-19; SARS-CoV-2; sotrovimab.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
There is no conflict of interest.
Figures


References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

