Synthesis and Conformational Analysis of Hydantoin-Based Universal Peptidomimetics
- PMID: 36226862
- PMCID: PMC10407853
- DOI: 10.1021/acs.joc.2c01903
Synthesis and Conformational Analysis of Hydantoin-Based Universal Peptidomimetics
Abstract
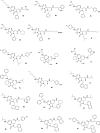
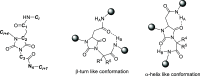
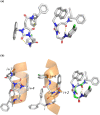
The synthesis of a collection of enantiomerically pure, systematically substituted hydantoins as structural privileged universal mimetic scaffolds is presented. It relies on a chemoselective condensation/cyclization domino process between isocyanates of quaternary or unsubstituted α-amino esters and N-alkyl aspartic acid diesters followed by standard hydrolysis/coupling reactions with amines, using liquid-liquid acid/base extraction protocols for the purification of the intermediates. Besides the nature of the α carbon on the isocyanate moiety, either a quaternary carbon or a more flexible methylene group, conformational studies in silico (molecular modeling), in solution (NMR, circular dichroism (CD), Fourier transform infrared (FTIR)), and in solid state (X-ray) showed that the presented hydantoin-based peptidomimetics are able to project their substituents in positions superimposable to the side chains of common protein secondary structures such as α-helix and β-turn, being the open α-helix conformation slightly favorable according to molecular modeling, while the closed β-turn conformation preferred in solution and in solid state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


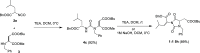
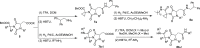

References
-
- Evans B. E.; Rittle K. E.; Bock M. G.; DiPardo R. M.; Freidinger R. M.; Whitter W. L.; Lundell G. F.; Veber D. F.; Anderson P. S.; Chang R. S.; Lotti V. J.; Cerino D. J.; Che T. B.; Kling P. J.; Kunkel K. A.; Springer J. P.; Hirshfield J. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J. Med. Chem. 1988, 31, 2235–2246. 10.1021/jm00120a002. - DOI - PubMed
- DeSimone R. W.; Currie K. S.; Mitchell S. A.; Darrow J. W.; Pippin D. A. Privileged Structures: Applications in Drug Discovery. Comb. Chem. High Throughput Screening 2004, 7, 473–493. 10.2174/1386207043328544. - DOI - PubMed
- Welsch M. E.; Snyder S. A.; Stockwell B. R. Privileged Scaffolds for Library Design and Drug Discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. 10.1016/j.cbpa.2010.02.018. - DOI - PMC - PubMed
- Alfano A. I.; Brindis M.; Lange H. Flow Synthesis Approaches to Privileged Scaffolds – Recent Routes Reviewed for Green and Sustainable Aspects. Green Chem. 2021, 23, 2233–2292. 10.1039/D0GC03883K. - DOI
-
- Pushpakom S.; Iorio F.; Eyers P. A.; Escott K. J.; Hopper S.; Wells A.; Doig A.; Guilliams T.; Latimer J.; McNamee C.; Norris A.; Sanseau P.; Cavalla D.; Pirmohamed M. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discovery 2019, 18, 41–58. 10.1038/nrd.2018.168. - DOI - PubMed
- Pillaiyar T.; Meenakshisundaram S.; Manickam M.; Sankaranarayanan M. A Medical Chemistry Perspective of Drug Repositioning: Recent Advances and Challenges in Drug Discovery. Eur. J. Med. Chem. 2020, 195, 11227510.1016/j.ejmech.2020.112275. - DOI - PMC - PubMed
-
- Shang S.; Tan D. S. Advancing Chemistry and Biology Through Diversity-Oriented Synthesis of Natural Product-Like. Curr. Opin. Chem. Biol. 2005, 9, 248–258. 10.1016/j.cbpa.2005.03.006. - DOI - PubMed
- Dandapani S.; Marcaurelle L. A. Current Strategies for Diversity-Oriented Synthesis. Curr. Opin. Chem. Biol. 2010, 14, 362–370. 10.1016/j.cbpa.2010.03.018. - DOI - PubMed
- Kim J.; Jung J.; Koo J.; Cho W.; Lee W. S.; Kim C.; Park W.; Park S. B. Diversity-Oriented Synthetic Strategy for Developing a Chemical Modulator of Protein-Protein Interaction. Nat. Commun. 2016, 7, 1319610.1038/ncomms13196. - DOI - PMC - PubMed
-
- Kaiser M.; Wetzel S.; Kumar K.; Waldmann H. Biology-Inspired Synthesis of Compound Libraries. Cell. Mol. Life Sci. 2008, 65, 1186–1201. 10.1007/s00018-007-7492-1. - DOI - PMC - PubMed
- Wetzel S.; Bon R. S.; Kumar K.; Waldmann H. Biology-Oriented Synthesis. Angew. Chem., Int. Ed. 2011, 50, 10800–10826. 10.1002/anie.201007004. - DOI - PubMed
- Garcia-Castro M.; Zimmermann S.; Sankar M. G.; Kumar K. Scaffold Diversity Synthesis and Its Application in Probe and Drug Discovery. Angew. Chem., Int. Ed. 2016, 55, 7586–7605. 10.1002/anie.201508818. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

