Randomized Phase 3 Trial of Ruxolitinib for COVID-19-Associated Acute Respiratory Distress Syndrome
- PMID: 36226977
- PMCID: PMC9668361
- DOI: 10.1097/CCM.0000000000005682
Randomized Phase 3 Trial of Ruxolitinib for COVID-19-Associated Acute Respiratory Distress Syndrome
Abstract
Objectives: Evaluate the safety and efficacy of the Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib in COVID-19-associated acute respiratory distress syndrome requiring mechanical ventilation.
Design: Phase 3 randomized, double-blind, placebo-controlled trial Ruxolitinib in Participants With COVID-19-Associated Acute Respiratory Distress Syndrome Who Require Mechanical Ventilation (RUXCOVID-DEVENT; NCT04377620).
Setting: Hospitals and community-based private or group practices in the United States (29 sites) and Russia (4 sites).
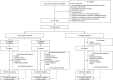
Patients: Eligible patients were greater than or equal to 12 years old, hospitalized with severe acute respiratory syndrome coronavirus 2 infection, and mechanically ventilated with a Pa o2 /F io2 of less than or equal to 300 mm Hg within 6 hours of randomization.
Interventions: Patients were randomized 2:2:1 to receive twice-daily ruxolitinib 15 mg, ruxolitinib 5 mg, or placebo, each plus standard therapy.
Measurements and main results: The primary endpoint, 28-day mortality, was tested for each ruxolitinib group versus placebo using a mixed-effects logistic regression model and one-tailed significance test (significance threshold: p < 0.025); no type 1 error was allocated to secondary endpoints. Between May 24, 2020 and December 15, 2020, 211 patients (age range, 24-87 yr) were randomized (ruxolitinib 15/5 mg, n = 77/87; placebo, n = 47). Acute respiratory distress syndrome was categorized as severe in 27% of patients (58/211) at randomization; 90% (190/211) received concomitant steroids. Day-28 mortality was 51% (39/77; 95% CI, 39-62%) for ruxolitinib 15 mg, 53% (45/85; 95% CI, 42-64%) for ruxolitinib 5 mg, and 70% (33/47; 95% CI, 55-83%) for placebo. Neither ruxolitinib 15 mg (odds ratio, 0.46 [95% CI, 0.201-1.028]; one-sided p = 0.029) nor 5 mg (odds ratio, 0.42 [95% CI, 0.171-1.023]; one-sided p = 0.028) significantly reduced 28-day mortality versus placebo. Numerical improvements with ruxolitinib 15 mg versus placebo were observed in secondary outcomes including ventilator-, ICU-, and vasopressor-free days. Rates of overall and serious treatment-emergent adverse events were similar across treatments.
Conclusions: The observed reduction in 28-day mortality rate between ruxolitinib and placebo in mechanically ventilated patients with COVID-19-associated acute respiratory distress syndrome was not statistically significant; however, the trial was underpowered owing to early termination.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Rein disclosed that she served as a consultant for AbbVie, Blueprint Medicines, Celgene, CTI BioPharma, and Novartis and as a site principal investigator on clinical trials involving ruxolitinib. Dr. Rein reports receiving research funding including salary support from Incyte Corporation for Ruxolitinib in Participants With COVID-19–Associated Acute Respiratory Distress Syndrome Who Require Mechanical Ventilation (RUXCOVID-DEVENT). Dr. Calero reports receiving grants from Incyte Corporation. Dr. Lodhi reports receiving grants and personal fees from Incyte Corporation and Theravance and nonfinancial support from Incyte Corporation. Drs. Daniel, Schaub, and O’Hayer disclosed they are employees of and own stock in Incyte Corporation. Dr. Hager reports receiving a research grant and salary support from Incyte Corporation for the conduct of the RUXCOVID-DEVENT trial, past salary support from the Embedded Precision in Acute Care Trials (EMPACT) Precision Medicine Network for participation in EMPACT Network and from the Marcus Foundation for the conduct of the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial, and other support from the Centers for Disease Control and Prevention (via subcontract with Vanderbilt University Medical Center). Dr. Theodoropoulos received funding from Incyte Corporation for the RUXCOVID-DEVENT trial. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242 - PubMed
-
- World Health Organization: COVID-19 Clinical Management: Living Guidance. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed March 24, 2021
-
- The ARDS Definition Task Force*: Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

