Incidence of Cutaneous Adverse Events With Phosphoinositide 3-Kinase Inhibitors as Adjuvant Therapy in Patients With Cancer: A Systematic Review and Meta-analysis
- PMID: 36227613
- PMCID: PMC9562095
- DOI: 10.1001/jamaoncol.2022.4327
Incidence of Cutaneous Adverse Events With Phosphoinositide 3-Kinase Inhibitors as Adjuvant Therapy in Patients With Cancer: A Systematic Review and Meta-analysis
Erratum in
-
Pervasive Data Errors in Abstract, Results, Figure 3, and Table 2.JAMA Oncol. 2023 Oct 1;9(10):1465. doi: 10.1001/jamaoncol.2023.3537. JAMA Oncol. 2023. PMID: 37651118 Free PMC article. No abstract available.
Abstract
Importance: The phosphoinositide 3-kinase (PI3K) pathway is among the most frequently activated pathways in human cancers. As the use of PI3K inhibitors for cancer treatment grows, there is increasing need for understanding the cutaneous effects associated with these therapies.
Objective: To systematically review the published literature reporting incidence of cutaneous adverse events with PI3K inhibitors and to provide pooled incidence estimates using meta-analysis.
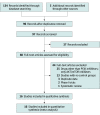
Data sources: This systematic review and meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. The literature search concerned entries through September 2021 in the following sources: PubMed, Cochrane registry, ClinicalTrials.gov, and evidence from the NHS UK and Trip medical database. To analyze PI3K inhibitors' cutaneous adverse events incidence, only randomized clinical trials (RCTs) were considered. The search strategy used the following keywords: (prevalence OR incidence OR epidemiology) and (phosphoinositide 3 kinase inhibitors OR PI3K inhibitors). No language restriction was applied. Analysis was conducted on July 1, 2022.
Study selection: Studies included phase 2 and phase 3 RCTs that reported incidence of cutaneous adverse events associated with use of PI3K inhibitors.
Data extraction and measures: Data extracted included sex, medication name and class, sample size, rash incidence, and grade. The bias risk was assessed by the Cochrane tool for risk of bias assessment in RCTs.
Main outcomes and measures: The primary outcome was incidence of PI3K inhibitor cutaneous adverse events (with 95% CIs) among the overall population and among subgroups. Between-study heterogeneity was assessed using the I2 statistic.
Results: The analysis found the incidence of PI3K inhibitor cutaneous events of any grade to be 29.30% in the intervention group, translating to a pooled odds ratio (OR) for incidence of cutaneous adverse events of any grades of 2.55 (95% CI, 1.74-3.75). Incidence of severe grade (grade ≥3) of rash in the intervention group was estimated to be 6.95%, yielding a pooled Peto OR of 4.64 (95% CI, 2.70-7.97). Subgroup analyses revealed that the incidence of severe cutaneous adverse events (grade ≥3) was higher with the use of Pan-class-1 PI3K inhibitors (OR, 6.67; 95% CI, 4.28-10.38) than isoform-selective PI3K inhibitors (OR, 6.37; 95% CI, 3.25-12.48).
Conclusions and relevance: This systematic review and meta-analysis identified an overall incidence of PI3K inhibitor cutaneous adverse events of any grade to be 29.30% with a pooled OR of 2.55; (95% CI, 1.74-3.75). These findings clarify the risk of cutaneous adverse events associated with this important class of anticancer therapies.
Conflict of interest statement
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

