Three-dimensional dose and LETD prediction in proton therapy using artificial neural networks
- PMID: 36227617
- PMCID: PMC9872814
- DOI: 10.1002/mp.16043
Three-dimensional dose and LETD prediction in proton therapy using artificial neural networks
Abstract
Purpose: Challenges in proton therapy include identifying patients most likely to benefit; ensuring consistent, high-quality plans as its adoption becomes more widespread; and recognizing biological uncertainties that may be related to increased relative biologic effectiveness driven by linear energy transfer (LET). Knowledge-based planning (KBP) is a domain that may help to address all three.
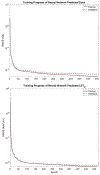
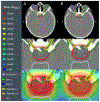
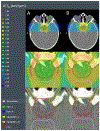
Methods: Artificial neural networks were trained using 117 unique treatment plans and associated dose and dose-weighted LET (LETD ) distributions. The data set was split into training (n = 82), validation (n = 17), and test (n = 18) sets. Model performance was evaluated on the test set using dose- and LETD -volume metrics in the clinical target volume (CTV) and nearby organs at risk and Dice similarity coefficients (DSC) comparing predicted and planned isodose lines at 50%, 75%, and 95% of the prescription dose.
Results: Dose-volume metrics significantly differed (α = 0.05) between predicted and planned dose distributions in only one dose-volume metric, D2% to the CTV. The maximum observed root mean square (RMS) difference between corresponding metrics was 4.3 GyRBE (8% of prescription) for D1cc to optic chiasm. DSC were 0.90, 0.93, and 0.88 for the 50%, 75%, and 95% isodose lines, respectively. LETD -volume metrics significantly differed in all but one metric, L0.1cc of the brainstem. The maximum observed difference in RMS differences for LETD metrics was 1.0 keV/μm for L0.1cc to brainstem.
Conclusions: We have devised the first three-dimensional dose and LETD -prediction model for cranial proton radiation therapy has been developed. Dose accuracy compared favorably with that of previously published models in other treatment sites. The agreement in LETD supports future investigations with biological doses in mind to enable the full potential of KBP in proton therapy.
Keywords: artificial intelligence; knowledge-based planning; neural networks; proton therapy.
© 2022 American Association of Physicists in Medicine.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to disclose.
Figures

Similar articles
-
Impact of range uncertainty on clinical distributions of linear energy transfer and biological effectiveness in proton therapy.Med Phys. 2020 Dec;47(12):6151-6162. doi: 10.1002/mp.14560. Epub 2020 Nov 14. Med Phys. 2020. PMID: 33118161
-
Dose averaged linear energy transfer optimization for large sacral chordomas in carbon ion therapy.Med Phys. 2024 Jun;51(6):3950-3960. doi: 10.1002/mp.17102. Epub 2024 May 2. Med Phys. 2024. PMID: 38696546
-
Comparing biological effectiveness guided plan optimization strategies for cranial proton therapy: potential and challenges.Radiat Oncol. 2022 Oct 22;17(1):169. doi: 10.1186/s13014-022-02143-x. Radiat Oncol. 2022. PMID: 36273132 Free PMC article.
-
Proton monoenergetic arc therapy (PMAT) to enhance LETd within the target.Phys Med Biol. 2020 Aug 19;65(16):165006. doi: 10.1088/1361-6560/ab9455. Phys Med Biol. 2020. PMID: 32428896
-
Quantitative analysis of dose-averaged linear energy transfer (LETd ) robustness in pencil beam scanning proton lung plans.Med Phys. 2022 May;49(5):3444-3456. doi: 10.1002/mp.15569. Epub 2022 Mar 7. Med Phys. 2022. PMID: 35194809
Cited by
-
Deep learning-based synthetic dose-weighted LET map generation for intensity modulated proton therapy.Phys Med Biol. 2024 Jan 5;69(2):025004. doi: 10.1088/1361-6560/ad154b. Phys Med Biol. 2024. PMID: 38091613 Free PMC article.
-
A Decision-Making Method for Photon/Proton Selection for Nasopharyngeal Cancer Based on Dose Prediction and NTCP.Cancers (Basel). 2025 Aug 11;17(16):2620. doi: 10.3390/cancers17162620. Cancers (Basel). 2025. PMID: 40867249 Free PMC article.
-
A deep learning model for predicting the modified micro-dosimetric kinetic model-based dose and the dose-averaged linear energy transfer for prostate cancer in carbon ion therapy.Phys Imaging Radiat Oncol. 2024 Nov 13;32:100671. doi: 10.1016/j.phro.2024.100671. eCollection 2024 Oct. Phys Imaging Radiat Oncol. 2024. PMID: 39624391 Free PMC article.
References
-
- Appenzoller LM, Michalski JM, Thorstad WL, Mutic S, Moore KL. Predicting dose-volume histograms for organs-at-risk in IMRT planning. Medical physics. 2012;39(12):7446–7461. - PubMed
-
- Moore KL, Appenzoller LM, Tan J, Michalski JM, Thorstad WL, Mutic S. Clinical implementation of dose-volume histogram predictions for organs-at-risk in IMRT planning. Journal of Physics: Conference Series. 2014;489:012055.
-
- Yuan L, Ge Y, Lee WR, Yin FF, Kirkpatrick JP, Wu QJ. Quantitative analysis of the factors which affect the interpatient organ-at-risk dose sparing variation in IMRT plans. Medical physics. 2012;39(11):6868–6878. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

