The entry of unclosed autophagosomes into vacuoles and its physiological relevance
- PMID: 36227834
- PMCID: PMC9562215
- DOI: 10.1371/journal.pgen.1010431
The entry of unclosed autophagosomes into vacuoles and its physiological relevance
Abstract
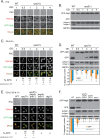
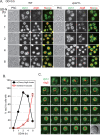
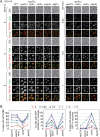
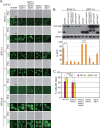
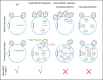
It is widely stated in the literature that closed mature autophagosomes (APs) fuse with lysosomes/vacuoles during macroautophagy/autophagy. Previously, we showed that unclosed APs accumulated as clusters outside vacuoles in Vps21/Rab5 and ESCRT mutants after a short period of nitrogen starvation. However, the fate of such unclosed APs remains unclear. In this study, we used a combination of cellular and biochemical approaches to show that unclosed double-membrane APs entered vacuoles and formed unclosed single-membrane autophagic bodies after prolonged nitrogen starvation or rapamycin treatment. Vacuolar hydrolases, vacuolar transport chaperon (VTC) proteins, Ypt7, and Vam3 were all involved in the entry of unclosed double-membrane APs into vacuoles in Vps21-mutant cells. Overexpression of the vacuolar hydrolases, Pep4 or Prb1, or depletion of most VTC proteins promoted the entry of unclosed APs into vacuoles in Vps21-mutant cells, whereas depletion of Pep4 and/or Prb1 delayed the entry into vacuoles. In contrast to the complete infertility of diploid cells of typical autophagy mutants, diploid cells of Vps21 mutant progressed through meiosis to sporulation, benefiting from the entry of unclosed APs into vacuoles after prolonged nitrogen starvation. Overall, these data represent a new observation that unclosed double-membrane APs can enter vacuoles after prolonged autophagy induction, most likely as a survival strategy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

