Deterioration of postural control due to the increase of similarity between center of pressure and smooth-pursuit eye movements during standing on one leg
- PMID: 36227896
- PMCID: PMC9560487
- DOI: 10.1371/journal.pone.0276119
Deterioration of postural control due to the increase of similarity between center of pressure and smooth-pursuit eye movements during standing on one leg
Abstract
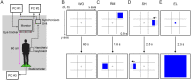
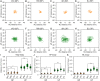
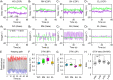
Upright postural control is regulated by afferent and efferent/reafferent visual mechanisms. There are two types of efferent and conjugate eye movements: saccades and smooth pursuits. Although postural control is improved by saccades, the effects of smooth pursuits on postural control are still debated, because the difficulties of postural and visual tasks differ in the previous research. Additionally, the mechanisms that interfere with postural control and smooth pursuit are not fully understood. To address these issues, we examined the effects of different patterns of smooth-pursuit eye movement on the path length of the center of pressure (COP) displacement under bipedal and unipedal standing conditions. The relative frequency and amplitude of the COP displacement were remarkably increased when uniform linear visual targets were presented during unipedal standing. In addition, dynamic time warping analysis demonstrated that the similarity between the displacement of the COP and eye movements was increased by the presentation of uniform linear visual targets with orientation selectivity during unipedal standing but not during bipedal standing. In contrast, the attenuation of similarity between the displacement of the COP and eye movements significantly decreased the path length, relative frequency, and amplitude of the COP displacement. Our results indicate that postural stability is deteriorated by the increase of similarity between the displacement of the COP and smooth-pursuit eye movements under unstable conditions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

