Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials
- PMID: 36227906
- PMCID: PMC9560143
- DOI: 10.1371/journal.pone.0276016
Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Whether vitamin D supplementation during pregnancy is beneficial to bone health and offspring growth remains controversial. Moreover, there is no universal agreement regarding the appropriate dose and the time of commencement of vitamin D supplementation during pregnancy.
Objective: We aimed to systematically review the effects of vitamin D supplementation during pregnancy on bone development and offspring growth.
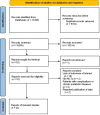
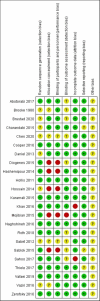
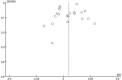
Methods: A literature search for randomized controlled trials (RCTs) was performed in 7 electronic databases to identify relevant studies about the effects of vitamin D supplementation during pregnancy on bone development and offspring growth from inception to May 22, 2022. A Cochrane Risk Assessment Tool was used for quality assessment. Vitamin D supplementation was compared with placebo or standard supplements. The effects are presented as the mean differences (MDs) with 95% CIs. The outcomes include bone mineral content (BMC), bone mineral density (BMD), bone area (BA), femur length (FL) and humeral length (HL); measurement indicators of growth, including length, weight and head circumference; and secondary outcome measures, including biochemical indicators of bone health, such as the serum 25(OH)D concentration. Additionally, subgroup analyses were carried out to evaluate the impact of different doses and different initiation times of supplementation with vitamin D.
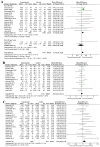
Results: Twenty-three studies with 5390 participants met our inclusion criteria. Vitamin D supplementation during pregnancy was associated with increased humeral length (HL) (MD 0.13, 95% CI 0.06, 0.21, I2 = 0, P = 0.0007) during the fetal period (third trimester). Vitamin D supplementation during pregnancy was associated with a significantly increased length at birth (MD 0.14, 95% CI 0.04, 0.24, I2 = 24%, P = 0.005) and was associated with a higher cord blood 25(OH)D concentration (MD 48.74, 95% CI 8.47, 89.01, I2 = 100%, P = 0.02). Additionally, subgroup analysis revealed that birth length was significantly higher in the vitamin D intervention groups of ≤1000 IU/day and ≥4001 IU/day compared with the control group. Prenatal (third trimester) vitamin D supplementation was associated with a significant increase in birth length, while prenatal (second trimester) vitamin D supplementation was associated with a significant increase in birth weight.
Conclusion: Vitamin D supplementation during pregnancy may be associated with increased humeral length (HL) in the uterus, increased body length at birth and higher cord blood 25(OH)D concentration. Evidence of its effect on long-term growth in children is lacking. Additional rigorous high-quality, long-term and larger randomized trials are required to more fully investigate the effects of vitamin D supplementation during pregnancy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. WHO antenatal care recommendations for a positive pregnancy experience: nutritional interventions update: vitamin D supplements during pregnancy[J]. 2020. - PubMed
-
- Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis[J]. JAMA pediatrics. 2018; 172(7): 635–645. doi: 10.1001/jamapediatrics.2018.0302 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

