Glaesserella parasuis serotype 5 breaches the porcine respiratory epithelial barrier by inducing autophagy and blocking the cell membrane Claudin-1 replenishment
- PMID: 36228044
- PMCID: PMC9595547
- DOI: 10.1371/journal.ppat.1010912
Glaesserella parasuis serotype 5 breaches the porcine respiratory epithelial barrier by inducing autophagy and blocking the cell membrane Claudin-1 replenishment
Abstract
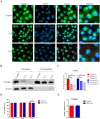
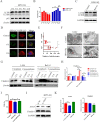
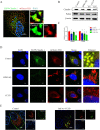
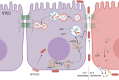
Glaesserella parasuis (G. parasuis), the primary pathogen of Glässer's disease, colonizes the upper respiratory tract and can break through the epithelial barrier of the respiratory tract, leading to lung infection. However, the underlying mechanisms for this adverse effect remain unclear. The G. parasuis serotype 5 SQ strain (HPS5-SQ) infection decreased the integrity of piglets' lung Occludin and Claudin-1. Autophagy regulates the function of the epithelial barrier and tight junction proteins (TJs) expression. We tested the hypothesis that HPS5-SQ breaking through the porcine respiratory epithelial barrier was linked to autophagy and Claudin-1 degradation. When HPS5-SQ infected swine tracheal epithelial cells (STEC), autophagosomes encapsulated, and autolysosomes degraded oxidatively stressed mitochondria covered with Claudin-1. Furthermore, we found that autophagosomes encapsulating mitochondria resulted in cell membrane Claudin-1 being unable to be replenished after degradation and damaged the respiratory tract epithelial barrier. In conclusion, G. parasuis serotype 5 breaks through the porcine respiratory epithelial barrier by inducing autophagy and interrupting cell membrane Claudin-1 replenishment, clarifying the mechanism of the G. parasuis infection and providing a new potential target for drug design and vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Cao Q, Wei W, Wang H, Wang Z, Lv Y, Dai M, et al. Cleavage of E-cadherin by porcine respiratory bacterial pathogens facilitates airway epithelial barrier disruption and bacterial paracellular transmigration. Virulence. 2021;12(1):2296–313. Epub 2021/09/07. doi: 10.1080/21505594.2021.1966996 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

