Defactinib, Pembrolizumab, and Gemcitabine in Patients with Advanced Treatment Refractory Pancreatic Cancer: A Phase I Dose Escalation and Expansion Study
- PMID: 36228156
- PMCID: PMC9772237
- DOI: 10.1158/1078-0432.CCR-22-0308
Defactinib, Pembrolizumab, and Gemcitabine in Patients with Advanced Treatment Refractory Pancreatic Cancer: A Phase I Dose Escalation and Expansion Study
Erratum in
-
Correction: Defactinib, Pembrolizumab, and Gemcitabine in Patients with Advanced Treatment Refractory Pancreatic Cancer: a Phase I Dose Escalation and Expansion Study.Clin Cancer Res. 2023 Nov 14;29(22):4698. doi: 10.1158/1078-0432.CCR-23-2993. Clin Cancer Res. 2023. PMID: 37960920 No abstract available.
Abstract
Purpose: Targeting focal adhesion kinase (FAK) renders checkpoint immunotherapy effective in pancreatic ductal adenocarcinoma (PDAC) mouse model. Defactinib is a highly potent oral FAK inhibitor that has a tolerable safety profile.
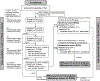
Patients and methods: We conducted a multicenter, open-label, phase I study with dose escalation and expansion phases. In dose escalation, patients with refractory solid tumors were treated at five escalating dose levels of defactinib and gemcitabine to identify a recommended phase II dose (RP2D). In expansion phase, patients with metastatic PDAC who progressed on frontline treatment (refractory cohort) or had stable disease (SD) after at least 4 months of standard gemcitabine/nab-paclitaxel (maintenance cohort) were treated at RP2D. Pre- and posttreatment tumor biopsies were performed to evaluate tumor immunity.
Results: The triple drug combination was well-tolerated, with no dose-limiting toxicities. Among 20 treated patients with refractory PDAC, the disease control rate (DCR) was 80%, with one partial response (PR) and 15 SDs, and the median progression-free survival (PFS) and overall survival (OS) were 3.6 and 7.8 months, respectively. Among 10 evaluable patients in the maintenance cohort, DCR was 70% with one PR and six SDs. Three patients with SD came off study due to treatment- or disease-related complications. The median PFS and OS on study treatment were 5.0 and 8.3 months, respectively.
Conclusions: The combination of defactinib, pembrolizumab, and gemcitabine was well-tolerated and safe, had promising preliminary efficacy, and showed biomarker activity in infiltrative T lymphocytes. Efficacy of this strategy may require incorporation of more potent chemotherapy in future studies.
©2022 American Association for Cancer Research.
Conflict of interest statement
Declaration of interests
The authors declare no competing interests for this manuscript.
Figures


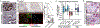
References
-
- Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(6):1326–36 doi 10.1158/1078-0432.CCR-17-3099. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

